Dataset of Non-Edible Calabash Chalk’s Bioactive Compounds: A Gas Chromatography-Mass Spectrometry Approach
| Received 20 Oct, 2023 |
Accepted 16 Mar, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Calabar stone, or calabash chalk, is a natural cosmetic composed of aluminum silicate hydroxide. Derived from fossilized seashells mixed with clay mud and elements like sand, wood ash and salt, it is used as a facial powder and antiperspirant. This study aims to investigate the phytochemical and bioactive components of calabash chalk used by herbalists in Imo State, Nigeria, for treating skin and wound infections. Materials and Methods: A chalk sample from herbalists was identified and deposited in the herbarium at Imo State University. The samples underwent drying, pulverizing and Soxhlet extraction. Extracts were obtained and bioactive compounds were analyzed using Agilent Technologies GC systems. Chemical components’ relative quantities were expressed as percentages based on chromatogram peak areas. Results: Solvent extracts of non-edible calabash chalk revealed the presence of various phytochemicals, including Hexadecanes, Methyl tetradecanoate, Tetradecanal, Hexadecanoic acid, Methyl ester, n-Hexadecanoic acid, known for antimicrobial, antioxidant and anticancer activities. Conclusion: The study establishes that calabash chalk utilized by herbalists contains bioactive components effective against various diseases. These components, identified and characterized using GC-MS technology, highlight calabash chalk’s potential as a source for medication development.
INTRODUCTION
Calabar stone, commonly known as calabash chalk, is an aluminum silicate hydroxide compound (Al2Si2O5(OH)4) belonging to the kaolin clay family1,2. It is a type of geographic material that is traditionally consumed by people (especially pregnant women) as a remedy for morning sickness, nausea and pleasure3. It is also used as a facial powder and as an antiperspirant when grounded4.
This natural cosmetic, composed of fossilized seashells, clay mud, sand, wood ash and salt, is available in various forms, such as bricks, pellets or powder. Beyond its conventional cosmetic applications, calabash chalk has garnered attention in Nigerian marketplaces, where vendors package and sell it. Herbalists also tout its medicinal properties for treating diverse skin infections5.
The significance of this study stems from the widespread and consistent use of calabash chalk by herbalists in Imo State, Nigeria, to combat an array of skin and wound infections, including erythrasma, impetigo, ecthyma, folliculitis, erysipelas and cellulitis (locally known as Ocha-ere). Despite these traditional practices, a scientific validation of the claimed therapeutic benefits is lacking.
To bridge this gap, current study employs Gas Chromatography-Mass Spectrometry (GC-MS) to comprehensively analyze the phytochemical and bioactive components of calabash chalk. The rationale behind this research lies in the necessity to reconcile traditional knowledge and scientific evidence, thereby establishing a foundation for understanding the therapeutic potential of calabash chalk.
By elucidating the chemical composition of calabash chalk; lead, arsenic and endrin amongst others6 this study can uncover potential synergistic effects among its constituents, leading to enhanced therapeutic efficacy. Moreover, understanding the mechanism of action underlying its medicinal properties can facilitate targeted drug development and formulation. This research endeavor holds promise not only for validating traditional practices but also for harnessing the therapeutic potential of natural remedies in addressing contemporary health challenges. Therefore, the current investigation seeks to identify and characterize the bioactive compounds within calabash chalk, shedding light on its pharmacological activities and bolstering its potential as a source for medication development.
Through this rigorous scientific examination, the study aims to provide empirical support for the traditional use of calabash chalk in treating various skin ailments, ultimately paving the way for its integration into modern medicinal practices.
MATERIALS AND METHODS
Sample collection and preparation: The study was conducted in Ohaji LGA, Imo State, Nigeria, from October 2022 to September 2023. A local herbalist in the specified location guided the sampling of calabash chalk (Nzu). Upon collection, a sample of the chalk underwent meticulous procedures, starting with identification, verification and acknowledgment by a plant taxonomist at Imo State University’s Department of Crop Science. The sample was then deposited in the herbarium of that institution with the voucher number DPS/IMSU/0628.
Calabash chalks (10 g) were obtained in their natural state and underwent a series of preparations. Initially, they were dried in an oven at 110°C to eliminate excess moisture. Subsequently, the dried chalks were pulverized in an acid-washed mortar and pestle. The extraction process involved utilizing the Soxhlet method.
Soxhlet extraction of materials: The samples were re-dried for 30 min in a 500 mL clean boiling flask inside a 110°C oven. It was then placed inside a desiccator to cool. The Soxhlet thimble was filled with 100 g of each sample. The extraction thimble was lightly blocked with cotton wool to aid in the extraction process and 300 mL of ethanol was added to the boiling flask to help in ethanol filtration. After being put together, the Soxhlet apparatus was placed in a 600°C reflux tank for 4 hrs. Carefully removing the thimble, the extract was then transferred to a volumetric flask for cooling. The volumetric flask’s contents were moved to a rotatory evaporator (Ecodyst, Apex, North Carolina, United States) to be separated into the solvent (n-hexane) and the oil.
Extraction of phytochemicals: One gram of extract was weighed and deposited in a test tube with 25 mL of ethanol. The test tube was put on a hotplate (FALC Instruments Treviglio (BG), Italy) at 600°C for 90 min. When the reaction period was up, the reaction product in the test tube was transferred to a separatory funnel. The tube was effectively cleaned by passing it through the funnel with 20 mL ethanol, 10 mL cold water, 10 mL hot water and 3 mL hexane. The extracts were combined three times and washed with a 10 mL ethanol aqueous solution containing 10% v/v ethanol. After drying with anhydrous sodium sulfate, the solvent was evaporated. The material was dissolved in 1000 μL pyridine, of which 200 μL was transferred to a vial for analysis.
GC-MS analysis: Agilent Technologies GC systems with GC-7890A/MS-5975C models (Agilent Technologies, Santa Clara, CA, USA) outfitted with HP-5MS columns (30 m length, 250 m diameter, 0.25 m film thickness) were utilized to investigate bioactive compounds from diverse extracts. For spectroscopic detection by GC-MS, an electron ionization system with high energy electrons was employed (70 eV). At a flow rate of 1 mL/min, the carrier gas was pure helium gas (99.995 percent purity). The initial temperature was set at 50-150°C, with a rise rate of 3°C/min and a hold time of 10 min. Finally, the temperature was raised to 300°C at a rate of 10°C per minute. One microliter of the obtained 1% extracts diluted with appropriate solvents was injected in a splitless mode. The relative quantity of chemical components included in each extract was expressed as a percentage based on the peak area obtained in the chromatogram.
Identification of chemical constituents: Bioactive compounds extracted from diverse extracts were identified using GC retention time on an HP-5MS column and spectra matching with computer software data of standards (Replib and Mainlab data of GC-MS systems). The National Institute of Standards and Technology (NIST) database, which contains over 62,000 patterns, was utilized to examine the mass spectrum GC-MS. The spectra of the unknown component were compared to the spectra of the known components in the NIST collection. The components in the test sample were identified by their name, molecular weight and structure4.
In this study, IT was calculated using a quasi-linear equation for temperature programmed retention index:
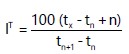 |
where, IT is the temperature-programmed retention index of the intriguing compound; tn, tn+1 and tx are the retention durations (in minutes) of the two standard n-alkanes with n and n+1 carbons, respectively and the compound of interest.
RESULTS AND DISCUSSION
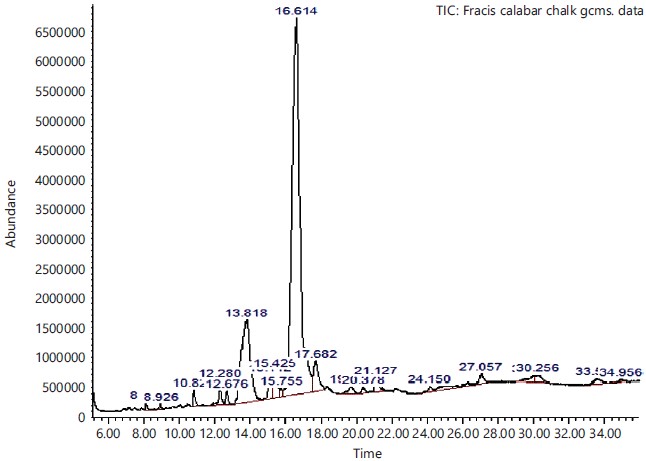
Phyto-component identities of the hexane GC-MS analyses of non-edible calabash chalk extracts: The GC-MS study revealed the presence of several phytochemicals in solvent extracts of non-edible calabash chalk (Fig. 1).
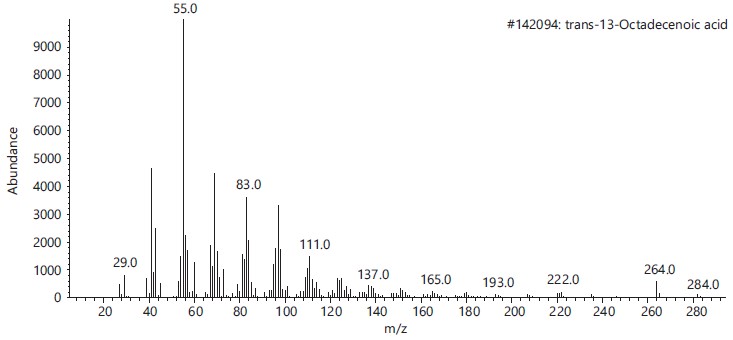
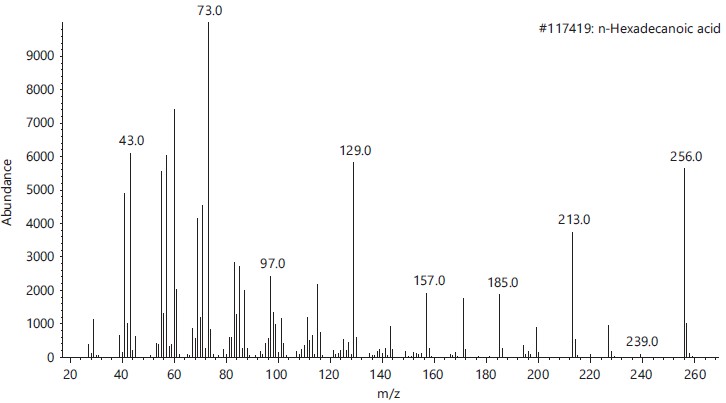
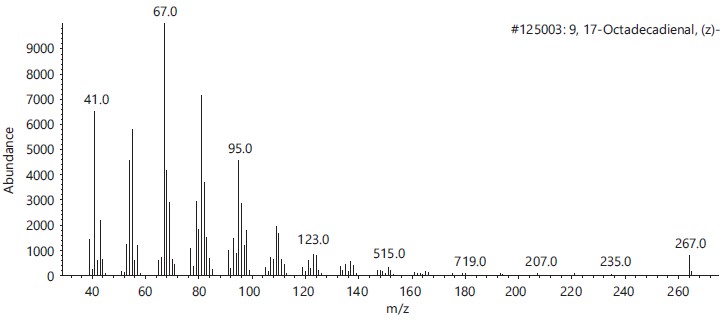
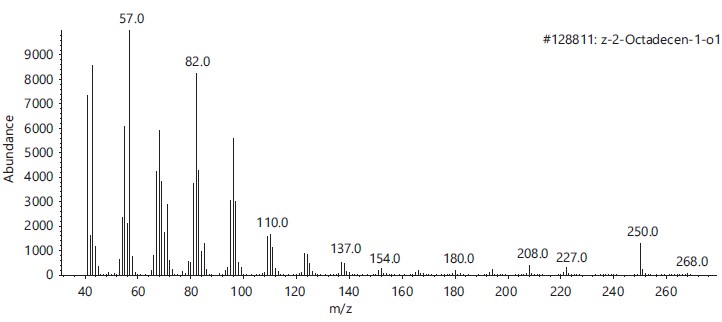
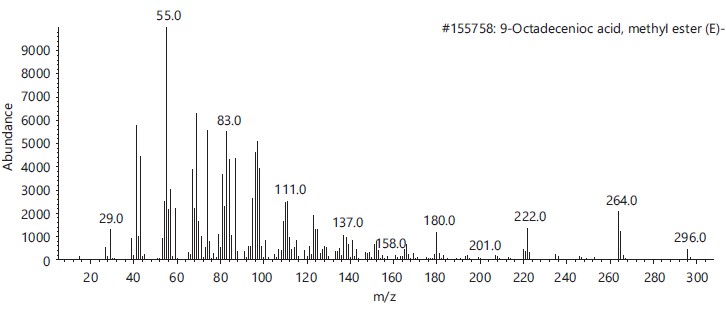
These compounds’ antimicrobial, antioxidant, anticancer and other pharmacological activities, as well as pesticidal and herbicidal properties, are documented in the NIST and PubChem databases (Table 1).The analysis revealed that the bioactive composition varied in different percentage components with trans-13-Octadecenoic acid (63.97% RT 16.614) having the highest percentage composition followed by, n-Hexadecanoic-acid (15.91%, RT 13.818), 9,17-Octadecadienal, (Z)- (3.10%, RT 17.682), Z-2-Octadecen-1-ol (2.54%, RT 27.057) while 9-Octadecenoic acid, Methyl ester with 2.74% and retention time of 15.425. Figure 2-6 depicts the mass spectrometry of these molecules.
|
| Table 1: | Phytocomponent identities of the hexane GC-MS analyses of non-edible | |||
Parameter |
Retention time |
Composition (%) |
Molecular formulae |
Molecular weight |
Activity |
| Hexadecanes | 8.12 |
0.34 |
C16H34 |
226.44 |
Antibacterial, antioxidant5 |
| Methyl tetradecanoate | 8.926 |
0.27 |
C15H30O2 |
242.4 |
Antibacterial6 |
| Tetradecanal | 10.821 |
0.68 |
C14H28O |
212.37 |
Bacterial and plant metabolite7 |
| Hexadecanoic acid, Methyl ester | 12.676 |
0.76 |
C17H34O2 |
270.4507 |
Antioxidant8 |
| n-Hexadecanoic-acid | 13.818 |
15.91 |
C16H32O2 |
256.42 |
Cytotoxic activities, anti-inflammatory and antioxidant9 |
| 9,12-Octadecadienoic acid (Z,Z)- | 15.142 |
1.7 |
C18H32O2 |
280.4 |
Antibacterial10 |
| 9-Octadecenoic acid, Methyl ester | 15.425 |
2.74 |
296.5 |
Antimicrobial activity10 | |
| Methyl stearate | 15.755 |
0.42 |
C19H38O2 |
282.5 |
Antifungal and antioxidant activity11 |
| trans-13-Octadecenoic acid | 16.614 |
63.97 |
C18H34O2 |
282.5 |
Antifungal12 |
| 9,17-Octadecadienal, (Z)- | 17.682 |
3.1 |
C18H31O2 |
264.4 |
Antimycobacterial13 |
| Oleic Acid | 16.692 |
0.8 |
C18H34O2 |
282.5 |
Antifungal activity12 |
| 9,12-Octadecadienoic acid | 20.378 |
0.46 |
C18H32O2 |
280.4 |
Antibacterial9 |
| 2-Methyl-Z,Z-3,13-octadecadienol | 21.127 |
1.21 |
C19H34O2 |
280.5 |
Antibacterial14 |
| - E 9- Tetradecenal | 24.150 |
0.48 |
C14H26O |
210.36 |
Antimicrobial10 |
| Z-2-Octadecen-1-ol | 27.057 |
2.54 |
C H O |
268.5 |
Antibiofilm15 |
| 1, Eicosadiene 19- | 30.006 |
0.96 |
C20H38 |
278.5 |
Antibacterial16 |
| Hexadecenoic acid, Z-11- | 30.256 |
1.06 |
C16H30O2 |
254.41 |
Desaturase17 |
| 6-Octadecenoic acid, (Z)- | 33.585 |
0.85 |
C18H34O2 |
282.5 |
Antimicrobial18 |
| n-Propyl 11-octadecenoate | 34.956 |
0.29 |
C21H40O2 |
324.5 |
Antifungal19 |
This study has delineated the bioactive constituents of calabash chalk, which has been the subject of numerous analyses by researchers concentrating on the core minerals of this natural product. The identified compounds-trans-13-Octadecenoic acid (63.97% RT 16.614), n-Hexadecanoic acid (15.91%, RT 13.818), 9,17-Octadecadienal (Z) (3.10%, RT 17.682), Z-2-Octadecen-1-ol (2.54%, RT 27.057) and 9-Octadecenoic acid, Methyl ester (2.74%, RT 15.425) are recognized as physiologically active molecules with potential for exploitation in medicinal development. This provides scientific validation for the usage of calabash chalk by herbalists in the study region, affirming its bioactive components as effective against a variety of diseases and infections1.
|
|
The GC-MS analysis, utilizing the database of the National Institute of Standards and Technology (NIST) containing over 62,000 patterns, alongside distinctive fragmentation patterns, facilitated the identification of specific classes of chemicals20-22. Table 1 illustrates the bioactive principles, incorporating their molecular formulas, molecular weights, retention times (RT), percentage content and distinct activities.
While there is a dearth of extensive studies employing GC-MS technology to evaluate the bioactive compounds in calabash chalk, existing literature has shed light on its mineral composition. Nafiu et al.23 revealed the mineral content of this geophagic material, uncovering its richness in calcium, magnesium, potassium, sodium, copper, manganese, iron, phosphorus and zinc, with the notable absence of lead (Pb). This is a pertinent finding given the concerns expressed by organizations in developed countries like the United Kingdom regarding Pb intake. The UK, for instance, has taken measures to prevent the import and use of substances with Pb levels significantly exceeding those typically found in most foodstuffs, which are usually well below 1 mg/kg23,24.
|
|
The variation in mineral concentration may be attributed to the geographical distribution of calabash chalk. This was supported by the research of Nafiu et al.23, who analyzed the mineral content of calabash chalk from Nigeria’s Eastern Region.
Furthermore, existing research corroborates the identified bioactive properties of certain compounds in calabash chalk. For example, Spiller et al.9 described the cytotoxic, anti-inflammatory and antioxidant properties of n-Hexadecanoic acid, while 9-Octadecenoic acid, Methyl ester, has been found to exhibit antimicrobial action10.
This study provides a comprehensive analysis of the bioactive components of calabash chalk, underscoring its potential for medicinal application. Furthermore, the scientific validation of calabash chalk’s medicinal properties can contribute to cultural preservation by acknowledging and preserving indigenous knowledge systems. It also opens avenues for collaboration between traditional healers and modern healthcare practitioners, fostering mutual respect and exchange of expertise. In essence, this study transcends the boundaries between traditional and modern medicine, highlighting the value of interdisciplinary approaches in advancing healthcare. Further research is warranted to fully explore the therapeutic benefits of these compounds and validate their efficacy in clinical settings.
|
CONCLUSION
The present study illuminated the rich bioactive composition of calabash chalk (commonly known as calabar stone) and demonstrated its potential therapeutic properties, especially in addressing skin and wound infections. Through GC-MS analyses, prominent phytochemicals such as trans-13-Octadecenoic acid, n-Hexadecanoic acid and 9,17-Octadecadienal, among others, were identified. The presence of these compounds provides scientific validation for the traditional use of calabash chalk by herbalists in the Imo State region of Nigeria. With reference to the database of the National Institute Standard and Technology (NIST) and other related studies, the identified bioactive components from the calabash chalk underscore the significance of this natural product in potential medicinal applications. Furthermore, given the distinct bioactive composition unveiled in this study, it seems pertinent to suggest that a botanical nomenclature be assigned to calabash chalk to better represent its medicinal potential. This will aid in standardizing its recognition in scientific communities and emphasize its relevance in therapeutic explorations.
SIGNIFICANCE STATEMENT
This pioneering study explores the uncharted territory of non-edible calabash chalk, shedding light on its bioactive components through cutting-edge Gas Chromatography-Mass Spectrometry (GC-MS) analysis. The herbalists’ successful utilization of calabash chalk in treating skin infections underscores the novelty and importance of this research. By unveiling previously undiscovered phytochemicals with potential therapeutic properties, this study lays the foundation for further exploration and underscores the relevance of traditional remedies in addressing contemporary healthcare challenges.
REFERENCES
- Nafiu, M.O., L.A. Alli and J.A. Aniah, 2016. Evaluation of calabash chalk effects on selected enzymes and histology of rat liver and kidney. Fountain J. Nat. Appl. Sci., 5: 1-11.
- Dean, J.R., M.E Deary, B.K Gbefa and W.C Scott, 2004. Characterisation and analysis of persistent organic pollutants and major, minor and trace elements in Calabash chalk. Chemosphere, 57: 21-25.
- Grigsby, R.K., B.A. Thyer, R.J. Waller and G.A. Johnston Jr., 1999. Chalk eating in middle Georgia A: Culture-bound syndrome of pica? Southern Med. J., 92: 190-192.
- Popoola, O.E., M.A. Bisi-Johnson, A. Abiodun and O.S. Ibeh, 2013. Heavy metal content and antimicrobial activities of some naturally occurring facial cosmetics in Nigeria. Ife J. Sci., 15: 637-644.
- Ramawat, K.G. and S. Goyal, 2008. The Indian Herbal Drugs Scenario in Global Perspectives. In: Bioactive Molecules and Medicinal Plants, Ramawat, K.G. and J.M. Merillon (Eds.), Springer, Berlin, Heidelberg, ISBN: 978-3-540-74603-4, pp: 325-347.
- Ekong, M.B., T.B. Ekanem, A.O. Sunday, A.N. Aquaisua and M. Akpanabiatu, 2012. Evaluation of calabash chalk effect on femur bone morphometry and mineralization in young Wistar rats: A pilot study. Int. J. Appl. Basic Med. Res., 2: 107-110.
- Yogeswari, S., S. Ramalakshmi, R. Neelavathy and J. Muthumary, 2012. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Global J. Pharmacol., 6: 65-71.
- Delamare, A.P.L., I.T. Moschen-Pistorello, L. Artico, L. Atti-Serafini and S. Echeverrigaray, 2007. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem., 100: 603-608.
- Spiller, G.A., C.D. Jensen, T.S. Pattison, C.S. Chuck, J.H. Whittam and J. Scala, 1987. Effect of protein dose on serum glucose and insulin response to sugars. Am. J. Clin. Nutr., 46: 474-480.
- Mohamed, E.A.A., A.M. Muddathir and M.A. Osman, 2020. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep., 10.
- Chandrasekaran, S., N.A. Nagendran, D. Pandiaraja, N. Krishnankutty and B. Kamalakannan, 2008. Bioinvasion of Kappaphycus alvarezii on corals in the Gulf of Mannar, India. Curr. Sci., 94: 1167-1172.
- Colombo, J., 2001. The development of visual attention in infancy. Annu. Rev. Psychol., 52: 337-367.
- Agoramoorthy, G., M. Chandrasekaran, V. Venkatesalu and M.J. Hsu, 2007. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol., 38: 739-742.
- Bachrach, G., A. Jamil, R. Naor, G. Tal, Z. Ludmer and D. Steinberg, 2011. Garlic allicin as a potential agent for controlling oral pathogens. J. Med. Food, 14: 1338-1343.
- Ayepola, O.O., 2009. Evaluation of the antimicrobial activity of root and leaf extracts of Terminalia glaucescens. Adv. Nat. Appl. Sci., 3: 188-191.
- Singh, K., M. Panghal, S. Kadyan, U. Chaudhary and J.P. Yadav, 2014. Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J. Nanomed. Nanotechnol., 5.
- Achi, N.K. and O.C. Ohaeri, 2015. GC-MS determination of bioactive constituents of the methanolic fractions of Cnidoscolus aconitifolius. Br. J. Pharm. Res. Int., 5: 163-172.
- Shigematsu, R., T. Okura, M. Nakagaichi, K. Tanaka, T. Sakai, S. Kitazumi and T. Rantanen, 2008. Square-stepping exercise and fall risk factors in older adults: A single-blind, randomized controlled trial. J. Gerontol.: Ser. A, 63: 76-82.
- Brands, M., E.B. Cahoon and P. Dörmann, 2020. Palmitvaccenic acid (Δ11-cis-hexadecenoic acid) is synthesized by an OLE1-like desaturase in the arbuscular mycorrhiza fungus Rhizophagus irregularis. Biochemistry, 59: 1163-1172.
- Brondz, I., 2017. Super antibiotics: Part III. hyperforin, revision of the relative and absolute stereochemistry presented by Bystrov et al. Voice Publisher, 3: 15-24.
- Garcia, A. and C. Barbas, 2011. Gas Chromatography-Mass Spectrometry (GC-MS)-Based Metabolomics. In: Metabolic Profiling: Methods and Protocols, Metz, T.O. (Ed.), Humana Press, Totowa, New Jersey, ISBN: 978-1-61737-985-7 pp: 191-204.
- Háznagy-Radnai, E., S. Czigle and I. Máthé, 2007. TLC and GC analysis of the essential oils of Stachys species. J. Planar Chromatogr. Mod. TLC, 20: 189-196.
- Nafiu, M.O., M.A. Akanji and M.T. Yakubu, 2011. Effect of aqueous extract of Cochlospermum planchonii rhizome on some kidney and liver functional indicies of albino rats. Afr. J. Tradit. Complementary Altern. Med., 8: 22-26.
- Wedeen, R.P., D.K. Mallik, V. Batuman and J.D. Bogden, 1978. Geophagic lead nephropathy: Case report. Environ. Res., 17: 409-415.
How to Cite this paper?
APA-7 Style
Chukwuebuka,
I.F., Ifenyinwa,
C.C., Ifunanya,
O.S., Nkechinyere,
N.R., Ijeoma,
A.I. (2024). Dataset of Non-Edible Calabash Chalk’s Bioactive Compounds: A Gas Chromatography-Mass Spectrometry Approach. Asian Science Bulletin, 2(3), 221-228. https://doi.org/10.3923/asb.2024.221.228
ACS Style
Chukwuebuka,
I.F.; Ifenyinwa,
C.C.; Ifunanya,
O.S.; Nkechinyere,
N.R.; Ijeoma,
A.I. Dataset of Non-Edible Calabash Chalk’s Bioactive Compounds: A Gas Chromatography-Mass Spectrometry Approach. Asian Sci. Bul 2024, 2, 221-228. https://doi.org/10.3923/asb.2024.221.228
AMA Style
Chukwuebuka
IF, Ifenyinwa
CC, Ifunanya
OS, Nkechinyere
NR, Ijeoma
AI. Dataset of Non-Edible Calabash Chalk’s Bioactive Compounds: A Gas Chromatography-Mass Spectrometry Approach. Asian Science Bulletin. 2024; 2(3): 221-228. https://doi.org/10.3923/asb.2024.221.228
Chicago/Turabian Style
Chukwuebuka, Ihenetu, Francis, Chikwendu Chinwe Ifenyinwa, Okorondu, Sylvester Ifunanya, Nwabueze Rose Nkechinyere, and Anekwe, Ifeoma Ijeoma.
2024. "Dataset of Non-Edible Calabash Chalk’s Bioactive Compounds: A Gas Chromatography-Mass Spectrometry Approach" Asian Science Bulletin 2, no. 3: 221-228. https://doi.org/10.3923/asb.2024.221.228

This work is licensed under a Creative Commons Attribution 4.0 International License.




