Efficacy of Neem (Azadirachta indica) Leaf Extract Against Fungi Pathogen Associated with Watermelon (Citrullus lanatus) Spoilage
| Received 31 Oct, 2023 |
Accepted 18 Mar, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Watermelon (Citrullus lanatus) is a member of the Cucurbitaceae family native to Africa and has been cultivated since ancient times. Reports of chemo-resistance pathogens and health implications have stalled the application of agrochemicals for improving fruit production. This led to the isolation, purification, identification of pathogens and use of Azadirachta indica leaf extract to inhibit pathogenic growth responsible for post-harvest deterioration of watermelon (Citrullus lanatus). The study aims to isolate, purify and determine growth rate, zone of inhibition and colony count of fungi pathogen of watermelon using standard mycological techniques to ascertain the effectiveness of Azadirachta indica leaf extract on the pathogen. Materials and Methods: Sample collection and media preparation, culture and purification of isolates, pathogenicity test, as well as neem leaf aqueous extract, were conducted using standard pathological methods in this research, after which five fungi pathogens such as Aspergillus flavus, Fusarium oxysporum, Aspergillus niger, Rhizopus sp. and Saccharomyces sp. They were isolated from infested watermelon fruits and treated with 5, 10, 20 and 40 g concentrations of neem extracts where 5.4, 5.8, 6.1 and 6.6 cm inhibition rates were observed. Results: Aspergillus species appeared undeterred by the extract at these concentrations, but the overall inhibitory potential of neem leaf extracted was observed at 40 g inhibitory concentration at 50% concentration (IC50). This means that the higher the concentration of the neem extract, the more effective in inhibiting pathogenic diseases of watermelon. Findings revealed that at controlled doses, Azadirachta indica leaf extracts may be able to suppress post-harvest pathogenic fungus in watermelon. Conclusion: To get compounds with significant resistance to post-harvest rotting, bioactive components should be assessed via bioassay-guided isolation.
INTRODUCTION
Watermelon (Citrullus lanatus) is a member of the Cucurbitaceae family native to Africa and has been cultivated since ancient times. The fruit flesh of watermelons is watery, but typically hard-textured, pale-colored and bland or bitter1. The watermelon fruit has a deep green smooth thick exterior rind with grey or light green vertical stripes. There are now eight designated flesh colors in watermelon: White, salmon yellow, orange, crimson red, scarlet red, pale yellow, canary yellow and green2. Watermelon contains more than 91% water and up to 7% carbohydrates. It is rich in some of the major antioxidants, vitamin C and carotenoids such as lycopene and β-carotene that are responsible for the red, orange and colors of the watermelon, the lycopene content of watermelon has become very important for consumers, as recently lycopene has stimulated attention as a health-promoting antioxidant. The sweetness of watermelon is mainly due to a combination of sucrose, glucose and fructose. Sucrose and glucose account for 20-40% and fructose for 30-50% of total sugars in a ripe watermelon.
Watermelon cultivation is not unconnected with losses due to post-harvest diseases especially those of fungi origin3. Synthetic fungicides are typically used to control phytopathogenic fungi. Since fungicides constitute a threat to human health and the environment, their use has been strictly regulated. However, after years of use, these pathogenic fungi have become highly resistant in plants, necessitating the implementation of a control mechanism to overcome this, microbiologists and plant scientists are paying more attention to phytochemicals because they are derived from plant components and have been shown to be safe for human health and biodegradable against microorganisms4,5.
As a result, it is inevitable to isolate, purify and identify the pathogenic fungus linked to the post-harvest degradation of watermelon fruits and to employ various plant leaf extraction techniques and disease management strategies to treat the phytopathogens. Therefore, this study aims to Isolate, purify and determine the growth rate, zone of inhibition and colony count of the fungi pathogen of watermelon using standard mycological techniques to ascertain the effectiveness of Azadirachta indica leaf extract on the fungi pathogen.
MATERIALS AND METHODS
Sample collection: Fresh skinned watermelon fruits were obtained from Anyigba’s main market. Samples were collected for 6 months (March to August, during the wet season of 2022) during the research and transported immediately to the Plant Science and Biotechnology Research Laboratory of Prince Abubakar Audu University, Anyigba, Kogi State. The sampled fruits were rinsed and air-dried at room temperature. The leaves of neem (Azardirachta indica) were obtained from Prince Abubakar Audu University agricultural farm. The leaves were rinsed with double distilled water (ddw) and were air-dried, pulverized and sieved (mesh size of 20 μm), 100 g of the dried leaves were measured and stored in an air-tight sample container at 4°C until ready for use.
Isolation of pathogen: About 39 g of potato dextrose agar (PDA) was weighed and dissolved in 1000 mL of distilled water, 1 mL of Tetracycline was prepared and transferred to a set of petri dishes, followed by sterilization at 121°C for 15 min. The infected fruits portion was sterilized using 70% ethanol, after which the infested fruits were scrapped off using a sterile scalpel and then transferred aseptically onto the agar media prepared6.
Identification of pathogen: The Identification and quantification of the pure isolates were investigated and observed at the end of incubation. Then selection of representative colonies and sub-cultured, until pure isolate culture is obtained and maintained on the PDA slant and stored. Pure culture was sub-cultured from the isolate obtained from the watermelon and maintained on a petri dish and daily readings were taken to identify the representative colonies and growth rate of fungal mycelia after 7 days7.
Pathogenicity test: The 70% of ethanol was used to disinfect fresh watermelon after it had been cleaned with distilled water. With the use of a sterile cork borer and the fungus mycelia, 4 mm diameter columns were extracted from the fruits (pure culture of the isolates). With the help of a sterile inoculating loop, the discs were taken out and put in fruit columns (a fruit column for a disc column). To stop other organisms from getting contaminated, the fruit skin columns were covered with vaseline gel and sealed. The inoculated watermelon fruit was kept on the laboratory bench for 21 days at a room temperature of 25°C. After the inoculation period, the watermelon fruit was cut across using a sterilized knife along the plane of inoculation and the rot depth was accessed.
Preparation of plant aqueous extract: The cold water extraction method was used for the preparation of the plant extracts. Fresh neem leaves were washed thoroughly with double distilled water and dried on the laboratory desk, it was pulverized and sieved to get a powder form, before being weighed at 5, 10, 20 and 40 g, respectively. The soluble powdered ingredient of the plant material was then extracted by adding 100 mL of distilled water to the weighed plant material. This was left for 24 hrs and then subsequently filtered through filter paper placed in the funnel under aseptic condition. This preparation gave 5, 10, 20 and 40% crude aqueous extract8.
Bioassay of plant extracts on the fungi isolates: Potato Dextrose agar medium was used; this was carried out in a sterile petri dish of 8.6 cm diameter. Containing solidified PDA medium. The 1 mL of 5, 10, 20 and 40% of the extract preparation was separately spread thinly on the surface of the PDA in the Petri dishes. A disc of 4 mm diameter (using a sterile cork borer) from each pure cultured fungi isolate was aseptically transferred and inoculated on the thin film of extract formed on the PDA. The organism was swabbed evenly on the extract of different concentrations (5, 10, 20 and 40%/disc). A replica was set for each treatment and control disc was set up with sterile distilled water. The treatment and control were both incubated at room temperature using the method of Amadioha and Uchendu9.
The percentage inhibition of fungi isolates by the plant extract at different concentrations was determined using Amadioha and Uchendu9:
| • | dc: Average diameter of fungal colony in the control | |
| • | dt: Average diameter of fungal colony in the treatment |
RESULTS AND DISCUSSION
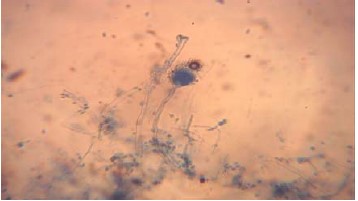
Results of pathogenic fungi studied indicated the presence of Aspergillus niger, Aspergillus flavus (Fig. 1), Fusarium oxysporum, Rhizopus sp. and Saccharomyces sp. Isolated and identified in deteriorated watermelon fruits as shown in Table 1. The in retro assessment and authenticity of these pathogen organisms were carried out using the pathogenicity test as employed by Al-Jaradi et al.10.
|
|
| Table 1: | Pathogenicity of fungi on watermelon | |||
| Fungi pathogen | Observed effects |
| Aspergillus niger | Fruits appeared water-soaked and soft, disease developed rapidly through fruit tissue resulting in total rot and exudation of liquids |
| Aspergillus flavus | A uses soft rot spoilage and the infected area turns brown and water-soaked |
| Fusarium sp. | Pathogen causes soft rot in the affected area |
| Rrhizopus sp. | Pathogen causes soft rot and water-soaked in the infected area, resulting in total rot in whole fruit |
| Saccharomyces sp. | A pathogen causes watery and soft rot in the infected area |
| Table 2: | Pathogenicity test showing the mean rot depth caused by pathogenic fungi | |||
| Fungi inoculated | Rot depth (mm) |
Rot depth |
Mean rot depth (mm) |
| Aspergillus niger | 30 |
31 |
30 |
| Aspergillus flavus | 29 |
30 |
30 |
| Fusarium oxysporum | 28 |
29 |
28 |
| Rhizopus stolani sp. | 34 |
34 |
34 |
| Saccharomyces sp. | 33 |
33 |
33 |
| Control | 00 |
00 |
00 |
| Table 3: | Mean radial growth (cm) of isolated fungi from rotted watermelon on PDA | |||
| Pathogen | Mean |
t-value |
Significant (2-tailed) |
| Aspergillus niger | 1.07 |
3.02 |
2.3×10–2 |
| Aspergillus flavus | 1.33 |
3.26 |
1.7×10–2 |
| Fusarium oxysporum | 1.93 |
3.84 |
9×10–2 |
| Rhizopus stolani | 2.13 |
3.9 |
8×10–2 |
| Saccharomyces sp. | 2.3 |
4.02 |
7×10–2 |
| Using the method of Amadioha and Uchendu9 | |||
| Table 4: | Percentage Inhibition rate of Azadrachta indica leaf extract on fungi pathogen | |||
Aspergillus niger |
Aspergillus flavus |
Fusarium oxysporum |
Rhizopus sp. |
Saccharomyces sp. |
||||||||||||||||
| Conc. (%) Azadirachta indica |
dc |
dt |
dc-dt |
Inhibit (%) dc-dt/dc×100 |
dc |
dt |
dc-dt |
Inhibit (%) dc-dt/dc×100 |
dc |
dt |
dc-dt |
Inhibit (%) dc-dt/dc×100 |
dc |
dt |
dc-dt |
Inhibit (%) dc-dt/dc×100 |
dc |
dt |
dc-dt |
Inhibit (%) dc-dt/dc×100 |
| 5 g | 4.3 |
0.1 |
4.2 |
97.67 |
4.3 |
0.6 |
0.6 |
86.05 |
4.3 |
0.1 |
42 |
97.67 |
4.3 |
4.3 |
0.0 |
0.00 |
4.3 |
0.3 |
4 |
93.02 |
| 10 g | 4.3 |
0.1 |
4.2 |
97.67 |
4.3 |
1.0 |
3.3 |
76.74 |
4.3 |
0.1 |
42 |
97.67 |
4.3 |
4.3 |
0.0 |
0.00 |
4.3 |
0.3 |
4 |
93.02 |
| 20 g | 4.3 |
0.1 |
4.2 |
97.67 |
4.3 |
1.5 |
2.8 |
65.12 |
4.3 |
0.1 |
42 |
97.67 |
4.3 |
4.3 |
0.0 |
0.00 |
4.3 |
0.3 |
4 |
93.02 |
| 40 g | 4.3 |
0.1 |
4.2 |
97.67 |
4.3 |
1.8 |
2.5 |
58.14 |
4.3 |
0.1 |
42 |
97.67 |
4.3 |
4.3 |
0.0 |
0.00 |
4.3 |
0.3 |
4 |
93.02 |
Table 2 below indicates the pathogenicity test showing the measured depth of the rot caused by the pathogen on watermelon fruit where Rhizopus sp. had the highest rot depth recorded while Fusarium oxysporum recorded the least.
The radical growth of Saccharomyces sp. represented in Table 3 completely covered the Petri-dishes on the 7th day of incubation (4.02 cm), followed by Rhizopus stolonifer (3.90 cm) and Fusarum oxysporum (3.84 cm) and Aspergillus flavus (3.26 cm, Fig. 2), while Aspergillus niger recorded the least (3.02 cm).
The percentage inhibition of fungi isolates by the plant extracted at different concentration.
| Table 5: | Fungi identified in deteriorated watermelon fruits | |||
| Plate | Characteristics or features | Probable fungus |
| 1A | It has fat yellowish-green becoming dark yellow-green conidiophores Conidial heads typically radiate, later splitting into several loose columns, Yellow-green becoming dark yellow-green conidiophores hyaline and coarsely roughened conidia globose to subglobose |
Aspergillus flavus |
| 2A | Serial mycelium sparse or floccose, becoming fatty, with whitish or peach, usually with purple tines, moving intensely near the medium surface Micro-conidia septate, borne on lateral, simple (often reduced) phial ides or on short branched conidiophores variable in shape and size, ovoid-ellipsodac clindrical, straight or slightly curled |
Fusarium oxysporum |
| 3A | Dense and floccose sometimes leathery, greyish white, cream to bluff, conidia slume formed in sporodochia or pionotes Micro-conidia usually abundant ovoid or oblong chlamydospores hyaline, smooth or rough walled, glucose to ovoid |
Aspergillus niger |
| 5A | Sporangiospores are singly or in groups from nodes directly above the rhinos and apophytes, columnetallate, multispored, geneally globose sporangium orangiospores are globose, one celled, hyaline to brown and striate in many species’ colonies fast growing and cover an agar surface with dense cottony growth that is at first white, becoming grey or yellowish |
Rhizopus sp. |
| 6A | Colonies of Saccharomyces grow rapidly and mature in three days They are flat, smooth, moist, glistening or dull, are cream or dark in color |
Saccharomyces sp. |
By using formula:
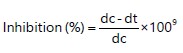 |
Where:
• |
dc = Average diameter of fungal colony in the control |
|
• |
dt = Average diameter of fungal colony in the treatment |
Table 4 below shows the percentage inhibition rate of A. indica leaf extract on the isolated fungi pathogens. The maximum inhibitory potential of the extract in the method of Amadioha and Uchendu9 was highest at 5 g/dm3 while the minimum value was observed at 40 g/dm3. There was no inhibition observed throughout the concentration’s treatment with Rhizopus sp.
From the study, pure phytopathogenic fungi (Aspergillus Niger, Aspergillus flavus, Fusarium oxysporum, Rhizopus ssp, Saccharomyces ssp) were isolated and identified in deteriorated watermelon fruits according to Koch postulate (Table 5). The retro assessment and authenticity of these pathogenic organisms were carried out using a pathogenicity test. Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Rhizopus sp and Saccharomyces spp.
DISCUSSION
The result of the pathogenicity test (Table 2) revealed that the fungi isolated from the spoilt watermelon fruits in this study were able to induce the same disease symptoms present in healthy fruit with the same fungi re-isolated from the inoculated healthy fruit. Thus, it shows that fungi were responsible for the spoilage of fruits.
There appeared no significant inhibition of Rhizopus throughout the treatment, this could be due to various factors including pathogen virulence as well as environmental interactions at the time of the experiment. This was also noted by Ibrahim et al.11 that Rhizopus spp. can grow over a wide range of temperatures, ranging from 15 to 40°C, with the optimal growth temperature of about 30°C. There doesn’t seem to be much significant inhibition on the pathogen tested except for Aspergillus flavus (Fig. 1) with an inhibition percentage of 58.14% as compared to others while Rhizopus indicated no inhibition whatsoever. This was in conformity with Bashir et al. 12 who worked on the effect of sodium carbonate and sodium chloride for the control of black rot disease of Mangifera indica caused by Aspergillus niger, much resistance was also observed by the pathogen even though conidia germination is hindered. Fulton, et al.13 also worked on the fungi diseases of watermelon and found Alternaria leaf blight (Alternaria cucumerina), Anthracnose (Colletotrichum orbiculare), Cercospora leafspot (Cercospora citrullina), downy mildew (Pseudoperonospora cubensis), Fusarium wilt (Fusarium oxysporum), Gummy stem blight (Didymella bryoniae) and powdery mildew (Podosphaera xanthii) affecting the growth of watermelon. He also opined that disease severity is high in areas where there is frequent rainfall and temperatures are high, a characteristic nature of Anyigba where this research was carried out. Alhaji et al.14 isolated the fungi associated with the rot of watermelon fruit in Sokoto metropolitan, Sokoto State, Nigeria and were able to isolate and identified Aspergillus flavus and Aspergillus niger. Rhizopus stolonifer and Mucor spp. and concluded that these pathogens confer great concerns to public health.
Fusarium oxysporum has also been implicated as the major causal organism of vascular wilt as well as the most important pathogen that causes serious yield loss yearly15,16. From this research, the treatment with 5 g concentration of neem doesn’t show any form of inhibition throughout the pathogens tested, however, the highest percentage inhibition of 58.14% was observed with 40 g treatment of the pathogen especially Aspergillus flavus (Fig. 1). This indicated that the higher the concentration of the neem extract, the more likely its ability to inhibit pathogenicity of a pathogen given that other environmental effects are within a controlled limit. This had also been suggested by Kutama et al.7, who worked on the in vitro inhibitory potential of Trichoderma species on Fusarium oxysporum f. sp vasinfectum the causal organism of vascular wilt of cotton (Gossypium hirsutum L.) in the Nigerian Sudan Savanna. Aspergillus niger appeared to have the least radial growth while Rhizopus solani had the highest mean radial growth (Table 3).
Fusarium diseases have been implicated to be a significant hindrance to food and plant production and are very difficult to manage, especially soil-borne diseases caused by F. oxysporum17. Fusariums are mycotoxin-producing fungi of great economic importance as they cause many diseases in man and other animals when infected food products are consumed. Some of the mycotoxins produced by Fusarium include FB1 and trichothecenes18. The effects of these mycotoxins on animals depend on the quantity of mycotoxin intake according to Bouhet and Oswald19 and after intake, they arrive at the gastrointestinal epithelial cell layer which is covered by the mucous secreted from goblet cells.
Fusarium species according to Sharma and Marques20, have diverse ecological functions ranging from saprophytes, endophytes and animal and plant pathogens. The major losses of watermelon postharvest in Kogi State had been a major setback to many farmers and traders alike in this axis, it is, therefore, imperative to employ several control measures for the management of these fungi pathogens.
Furthermore, growing disease-resistant species to combat this disease is a more appropriate strategy because synthetic fungicides are not a true way to prevent fungi-related diseases due to their harmful effects on the ecosystem and the environment 21. This study was carried out to determine the efficacy of neem (Azadirachta indica) leaf extract associated with fungi pathogen affecting watermelon obtained from Anyigba market. The neem (Azadirachta indica) tree has gained global recognition due to its numerous medical benefits. Antifungal, immunomodulatory, anti-inflammatory, antihyperglycemic, antiulcer, antimalarial, antibacterial, antioxidant, antimutagenic and anticarcinogenic qualities have been shown for neem leaf and its compounds22.
Agricultural and chemical precautions cannot be overly successful in the management of fungi-related diseases in plants. The most effective way to address fungal-induced plant diseases is through the application of biological control techniques. such as the extracts of neem that have been proven by the findings of this research work to be of promising, efficient, cheap and eco-friendly means of protecting plants and plant products.
CONCLUSION
To overcome the alarming problem of fungal infections or contaminations on fruits, the discovery of phytochemicals against new targets is a matter of urgency. Thus, A. indica could become promising natural antifungal agents with potential applications in the pharmaceutical industry for controlling pathogenic fungi. The plant extracts in the study (A. indica) could be used to inhibit rot fungi of watermelon fruit at controlled concentrations, thereby increasing the shelf life and high market value of watermelon fruit.
SIGNIFICANCE STATEMENT
Watermelon (Citrullus lanatus) is a large annual plant with long, trailing or climbing stems and belongs to Cucurbitaceae. The mineral and nutritional compositions revealed bioactive constituents such as phenol, crude fiber, flavonoids, phosphorus, sodium, manganese, potassium, copper, calcium, zinc, iron, fat content, lipid, crude ash and moisture content. However, fungi pathogens are a major deterrent to the yield, productivity, shelf life and market value of watermelon especially in this central part of Nigeria. This research has unveiled a new, cheap and environmentally friendly way of controlling the diseases of watermelon via green and friendly sources. This will boost the economy of this region and the entire country and the world at large once these findings are duly accepted and harnessed.
REFERENCES
- Paris, H.S., 2015. Origin and emergence of the sweet dessert watermelon, Citrullus lanatus. Ann. Bot., 116: 133-148.
- Choudhary, H., K. Padmanabha, G.S. Jat and T.K. Behera, 2023. Challenges of Traditional Breeding in Watermelon. In: The Watermelon Genome, Dutta, S.K., P. Nimmakayala and U.K. Reddy (Eds.), Springer, Cham, Switzerland, ISBN: 978-3-031-34716-0, pp: 85-130.
- Ahmad, B., M. Mehdi, Abdul Ghafoor and H. Anwar, 2018. Value chain assessment and measuring export determinants of citrus fruits in Pakistan: An analysis of primary data. Pak. J. Agri. Sci., 55: 685-692.
- Behbahani, M., M. Shanehsazzadeh, Y. Shokoohinia and M. Soltani, 2013. Evaluation of anti-herpetic activity of methanol seed extract and fractions of Securigera securidaca in vitro. J. Antivirals Antiretrovirals, 5: 72-76.
- Salin, N.S.M., W.M.M. Saad, H.R. Abdul Razak and F. Salim, 2022. Effect of storage temperatures on physico-chemicals, phytochemicals and antioxidant properties of watermelon juice (Citrullus lanatus). Metabolites, 12.
- Oladeji, S.O. and K.A. Odelade, 2016. Screening, isolation and identification of microorganisms from petrochemical contaminated environment. Braz. J. Biol. Sci., 3: 201-208.
- Aliyu, U., A.S. Kutama, S. Zafar, A.A. Bashir and M.M. Hadiza, 2022. In vitro inhibitory potential of Trichoderma species on Fusarium oxysporum f.sp Vasinfectum the causal organism of vascular wilt of cotton (Gossypium hirsutum L.) in the Nigerian Sudan Savanna. UMYU Scientifica, 1: 122-126.
- Ramirez-Rodrigues, M.M., M.L. Plaza, A. Azeredo, M.O. Balaban and M.R. Marshall, 2011. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci., 76: C428-C435.
- Amadioha, A.C. and P.N. Uchendu, 2003. Post harvest control of tomato fruit rot caused by Fusarium solani with extracts of Azadirachta indica. Discovery Innovation, 15: 83-86.
- Al-Jaradi, A., I. Al-Mahmooli, R. Janke, S. Maharachchikumbura, N. Al-Saady and A.M. Al-Sadi, 2018. Isolation and identification of pathogenic fungi and oomycetes associated with beans and cowpea root diseases in Oman. PeerJ, 6.
- Ibrahim, A.S., B. Spellberg, T.J. Walsh and D.P. Kontoyiannis, 2012. Pathogenesis of mucormycosis. Clin. Infect. Dis., 54: S16-S22.
- Bashir, A.A., T.I. Egbeja, M.M. Namadina, S.U. Umar, A. Aminu and J. Idakwo, 2022. Effects of sodium carbonate and sodium chloride on the control of black rot disease of Mangifera indica L. (Mango) caused by Aspergillus niger. J. Plant Sci., 17: 166-171.
- Fulton, J.C., B.S. Amaradasa, T.S. Ertek, F.B. Iriarte and T. Sanchez et al., 2021. Phylogenetic and phenotypic characterization of Fusarium oxysporum f. sp. niveum isolates from Florida-grown watermelon. PLoS ONE, 16.
- Alhaji, Y.I., S.Y. Lema, J. Ibrahim, A. Umar and M. Garba, 2020. Isolation and identification of fungi associated with rot of watermelon fruit in Sokoto metropolitan, Sokoto State, Nigeria. Int. J. Pathogen Res., 5: 78-83.
- Kutama, A.S., M. Adamu, H.U. Baita, S. Zafar and M.M. Hadiza, 2022. Review on the contributions of some human cultural practices to plant disease epidemiology. Dutse J. Pure Appl. Sci., 8: 12-20.
- Kareem, T.K., O.E. Ugoji and O.O. Aboaba, 2016. Biocontrol of Fusarium wilt of cucumber with Trichoderma longibrachiatum NGJ167 (Rifai). Microbiol. Res. J. Int., 16.
- Arie, T., 2019. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci., 44: 275-281.
- Quarta, A., G. Mita, M. Haidukowski, A. Santino, G. Mulè and A. Visconti, 2005. Assessment of trichothecene chemotypes of Fusarium culmorum occurring in Europe. Food Addit. Contam., 22: 309-315.
- Bouhet, S. and I.P. Oswald, 2005. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol., 108: 199-209.
- Sharma, L. and G. Marques, 2018. Fusarium, an entomopathogen-A myth or reality? Pathogens, 7.
- Askun, T., 2018. Introductory Chapter: Fusarium: Pathogenicity, Infections, Diseases, Mycotoxins and Management. In: Fusarium-Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers, Askun, T. (Ed.), IntechOpen, London, United Kingdom, ISBN: 978-1-78923-319-3, pp: 1-12.
- Iman, M., M. Taheri and Z. Bahari, 2022. The anti-cancer properties of neem (Azadirachta indica) through its antioxidant activity in the liver: its pharmaceutics and toxic dosage forms. A literature review. J. Complementary Integr. Med., 19: 203-211.
How to Cite this paper?
APA-7 Style
Ali,
B.A., Shehu,
K.A., Salisu,
S.M., Unoyiza,
U.S., Babalola,
B.J., Tijani,
K.B., Muhammad,
N.M., ,
A.F., Yusuf,
Z.J., Ftima,
N.A. (2024). Efficacy of Neem (Azadirachta indica) Leaf Extract Against Fungi Pathogen Associated with Watermelon (Citrullus lanatus) Spoilage. Asian Science Bulletin, 2(3), 249-257. https://doi.org/10.3923/asb.2024.249.257
ACS Style
Ali,
B.A.; Shehu,
K.A.; Salisu,
S.M.; Unoyiza,
U.S.; Babalola,
B.J.; Tijani,
K.B.; Muhammad,
N.M.; ,
A.F.; Yusuf,
Z.J.; Ftima,
N.A. Efficacy of Neem (Azadirachta indica) Leaf Extract Against Fungi Pathogen Associated with Watermelon (Citrullus lanatus) Spoilage. Asian Sci. Bul 2024, 2, 249-257. https://doi.org/10.3923/asb.2024.249.257
AMA Style
Ali
BA, Shehu
KA, Salisu
SM, Unoyiza
US, Babalola
BJ, Tijani
KB, Muhammad
NM,
AF, Yusuf
ZJ, Ftima
NA. Efficacy of Neem (Azadirachta indica) Leaf Extract Against Fungi Pathogen Associated with Watermelon (Citrullus lanatus) Spoilage. Asian Science Bulletin. 2024; 2(3): 249-257. https://doi.org/10.3923/asb.2024.249.257
Chicago/Turabian Style
Ali, Bashir, Alabi, Kutama Ahmad Shehu, Suleiman Mohammed Salisu, Umar Sabdat Unoyiza, Balogun Joshua Babalola, Kokori Bajeh Tijani, Namadina Murtala Muhammad, Abdulsalam Fiddausi , Zubairu Joseph Yusuf, and Ndah Abigail Ftima.
2024. "Efficacy of Neem (Azadirachta indica) Leaf Extract Against Fungi Pathogen Associated with Watermelon (Citrullus lanatus) Spoilage" Asian Science Bulletin 2, no. 3: 249-257. https://doi.org/10.3923/asb.2024.249.257

This work is licensed under a Creative Commons Attribution 4.0 International License.




