Male Reproductive Hormonal Activity of Synergistic Ingestion of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera in Male Rat Model
| Received 22 Oct, 2023 |
Accepted 30 Jan, 2024 |
Published 22 Feb, 2024 |
Background and Objective: Masculinity encompasses sexual potency as one of its determinants. The global rise in rates of sexual dysfunctionality in males has gained much interest in the world of science, hence, a need for ways to enhance and manage sexual dysfunctionality. This study evaluated the effects of the synergistic mixture of Cyperus esculentus (tiger nuts), Phoenix dactylifera (dates) andCocos nucifera (coconut), (STCD) on the reproductive hormones in a male rat model. Materials and Methods: Acute toxicity LD50 of STCD was carried out according to Organization for Economic Co-operation and Development (OECD) guidelines, afterwards, fifteen male albino rats were grouped into three groups with 5 rats in each group; Control, 200 and 400 mg/kg STCD. The rats were administered STCD orally for 24 hrs, for 21 days, with feed and water ad libitum. Blood samples were collected via cardiac puncture for biochemical analysis while the tests were harvested for histological examination. The IBM SPSS, Version 25 was used for statistical analysis. Results: Acute toxicity LD50 of STCD was observed to be ≥2404.2 mg/kg body weight. An increase in the serum concentration of Follicle-Stimulating Hormone (FSH), Luteinizing Hormone (LH) and testosterone were observed, while a decrease in prolactin was observed in the group administered STCD at 200 and 400 mg/kg STCD with values, 0.526±0.193, 1.166±0.440, 0.756±0.130, 0.536±0.217 and 1.018±0.741, 1.700±0.835, 0.874±0.516 and 0.284±0.627, respectively, when compared to the control. The histological examination showed numerous germ cells with normal spermatozoa for control and 200 mg/kg extract, while that of 400 mg/kg extract showed a spermatic cord containing mature spermatozoa. Conclusion: The results of this present study show that STCD had enhancing effects on the male reproductive hormones investigated, thus this study suggests the enhancing potency on male sexual libido and performance of STCD. This is also evidenced by the copulatory behavior observed in the experimental rats when administered STCD.
INTRODUCTION
The use of natural products in the enhancement, management and treatment of disease has been a global trend, with lots of recommendations and advocacy from the World Health Organization1. An aphrodisiac is seen as any substance that enhances sex drive or desire. In reality, any substance that increases erectile function, sexual performance and enjoyment has been considered as an aphrodisiac2,3. In males, the sexual reproductive hormones involved in its functioning are; Follicle-Stimulating Hormone (FSH), luteinizing hormone, testosterone and prolactin4. Testosterone has been reported to be responsible for promoting puberty and the integrity of the male reproductive system. The features of puberty in males such as muscle mass increase, voice deepening, spermatogenesis and fertility5,6.
The FSH in men stimulates testicular growth and helps produce a protein that plays a vital role in male fertility by aiding in the creation of normal sperm cells and maintaining them until they are ready to be released7. Luteinizing Hormone (LH) is a glycoprotein hormone that is co-secreted along with follicle-stimulating hormone by the gonadotrophin cells in the adenohypophysis (anterior pituitary). The LH release is stimulated by Gonadotropin-Releasing Hormone (GnRH) and inhibited by testosterone in males. In men, LH causes the Leydig cells of the testes to produce testosterone8. Prolactin (PRL) is mainly synthesized and secreted by the lactotroph cells of the pituitary9. Physiologic levels of prolactin in males enhance luteinizing hormone receptors in Leydig cells, resulting in testosterone secretion, which leads to spermatogenesis10.
Cyperus esculentus (tiger nut) has served as a food source for humans and animals due to its rich nutritional contents of fat, carbohydrates, minerals (calcium, iron, copper, cobalt, magnesium, fluorine, manganese, phosphorus, potassium copper, sodium, boron, sulfur, zinc and selenium), enzymes like catalases, lipases and amylases, which aid in the digestion of foods in the gut, reducing gas, indigestion and diarrhea11. It contains a considerable quantity of secondary metabolites such as tannins, saponins, phytate, oxalates and cyanogenic glycosides. Tiger nuts have been reported to improve the integrity of the heart and cardiovascular system. It also helps in ameliorating inflammation and malignancy. This attribute can be associated with the high content of soluble glucose in it12. Tiger nuts are high in insoluble fiber and arginine, which can help in avoiding constipation, lower blood sugar and maintain a healthy digestive system to prevent painful gas or bloating13,14. Allouh et al.15 in their study supported the hypothesis that Cyperus esculentus tubers have aphrodisiac activity, enhancing male sexual libido and performance. The observed improvements in copulatory behavior after the administration of tiger nuts could be partially attributed to increased serum testosterone levels in male rats.
Phoenix dactylifera (date palm) are sweet berries with a sugar content of more than 50%. Due to its high nutritional value, great yields and long life the date palm has been mentioned as the “tree of life”16,17. Date palms are rich in mineral nutrients such as; calcium, iron, copper, cobalt, magnesium, fluorine, manganese, phosphorus, potassium copper, sodium, boron, sulfur, zinc and selenium. They have also been found to have a high content of riboflavin, biotin, thiamine, ascorbic and folic acid which are essential for the body18,19. The presence of these compounds in different proportions could promote their nutraceutical potential. These nutraceutical potentials include antimicrobial activities, antioxidant capacities and anticancer. Date has been used since ancient times as an aphrodisiac and fertility enhancer in patients with sexual dysfunction or infertility. Rahmani et al.20 concluded that, Phoenix dactylifera may increase antioxidant enzymes that provide protection to hepatocytes against oxidative stress.
Cocos nucifera (coconut), belongs to the family Arecaceae commonly cultivated in many parts of the world. Gandhi et al.21 concluded from their experiment that treatment with coconut water inhibited crystal deposition in renal tissue as well as reduced the number of crystals in urine. The anti-diarrhea and electrolyte-boosting potency of coconut has been reported22. Zulaikhah et al.23 in their study observed that coconut water has the potency to prevent anemia and boost blood profile functioning. The antihypertensive potentials of coconut water were also reported by Airaodion et al.24.
Sexual dysfunction is a condition whose root cause is not fully understood, hence, there are limited treatments to combat the condition. Although some traditional remedies and herbal drugs have been used by patients with sexual dysfunction, a significant proportion of medicinal plants have not yet been scientifically evaluated. The mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera has been speculated to have effects on the male reproductive hormones but has not been scientifically proven, hence the purpose of this study. Some published reports on similar studies reported the health benefits of the STCD on hepatic and renal function25 and the lipid-lowering potency of the STCD26. This present study evaluated the male reproductive hormonal activity of the synergistic ingestion of aqueous extract of Cyperus esculentus (tigernut seeds), Phoenix dactylifera (dates) and Cocos nucifera (coconut) in male rat models.
MATERIALS AND METHODS
This study was undertaken at the Department of Biochemistry, Rivers State University, Port Harcourt, Nigeria from November, 2022 to March, 2023.
Plant material and preparation of extract: As described in previous publications of the joint project (Odinga et al.25 and Bouhlali et al.26), Fresh seeds of tiger nuts, dates and coconut were purchased from mile 1 market in Port Harcourt and authenticated at the herbarium of the Plant Science and Biotechnology Department of Rivers State University. Tiger nuts (400 g) were cleaned to remove impurities, washed and put in a bowl. The dates (120 g) were cut into two to remove the seeds, then washed and put in a bowl. The coconut (200 g) was removed from the shell, diced and washed. All were pulverized with a manual blender, adding 150 mL of water until a uniform mixture was obtained. The mixture was filtered using a sieve and the resulting filtrate was put into a bottle until use. This synergistic mixture was prepared daily for the 21 days of administration.
Acute toxicity studies (LD50) of STCD: The acute toxicity of STCD extract was determined by the Botham27 procedure outlined by Lorke28 as reported by Adefisayo et al.29. The rats were fasted overnight prior to experiment. The experiment was carried out in 2 phases: In Phase 1, 9 rats were used, they were grouped into 3, with 3 rats in each group. The STCD extract was administered via gavage at the doses of 10, 100 and 1000 mg/kg. In the second phase, 8 rats were divided into 4 groups of 2 rats each and they were administered with STCD extract by gavage at the doses of 850, 1700, 3400 and 6800 mg/kg. The general behavior of the animals was observed continuously for 1 hr after administration, then intermittently for 4 hrs and finally hourly for the next 24 hrs. The LD50 was determined using the formula25:
Where:
a |
= |
Least dose that killed any rat |
|
b |
= |
Highest dose that did not kill any rat |
Experimental protocol: After acclimatization of the experimental rats, they were properly grouped; Group 1: Experimental rats acting as the control group were administered normal rat feed and distilled water, Group 2: Experimental rats acting as the low dose, were administered 200 mg/kg of the STCD extract and Group 3: Experimental rats which was the high dose were administered 400 mg/kg of the STDC extract. The administration in both Group 2 and 3 was done orally using an oral gavage tube. The administration lasted for 21 days and animals were sacrificed.
Collection of blood samples: Sacrifice was done in three batches; the first was carried out after 7 days, the second after 14 days and the third after 21 days. Blood samples (5 mL) were collected through cardiac puncture from each rat and put in plain sample bottles for biochemical analysis of Prolactin, Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Testosterone using the methods as described by Odinga et al.30,31. The testes were carefully harvested from each animal for histological examination. Testes samples meant for histological analysis were immediately fixed at 10% normal saline.
Histological analysis: The testes of the rats were harvested and fixed in 10% formalin, dehydrated in graded alcohol, cleared in xylene and embedded in paraffin wax. The tissues were then cut into 2-3 mm thick sections by a microtome, fixed on the slides and stained with hematoxylin-eosin. The slides were examined under a light microscope (Olympus CH; Olympus, Tokyo, Japan) and photomicrographs were taken with a Leica DM 750 camera at ×100 magnification.
Statistical analysis: The results obtained were expressed as Mean±Standard Deviation. Data were analyzed using a one-way analysis of variance followed by post hoc test using Turkey’s Test and p-value less than 0.05 was considered statistically significant. The statistical analysis was performed with the aid of IBM SPSS, Version 25.
Ethical consideration: Handling and treatment of the rats were in conformation with the guidelines of the National Institute of Health as described by Odukanmi et al.32 for laboratory animal care and use. All animals in this study adhered to the Institutional Animal Ethical Committee according to guidelines given by Committee for Control and Supervision of Experiments on Animals (CPCSEA). This study received approval from the Ethical committee of Rivers State University.
RESULTS
The Acute Oral Toxicity test (LD50) of STCD was determined to be ≥2404.2 mg/kg body weight in adult male Wistar rat model.
Body weight analysis: Table 1 show the body and organ weight of the experimental animals in the various groups. The percentage mean body weight of the animals shows a decrease in the percentage mean body weight of the animal after the administration of the synergistic mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera when comparing the various groups. For control group 30.95 g, for 200 mg/kg STCD 8.39 g and 400 mg/kg STCD 2.86 g.
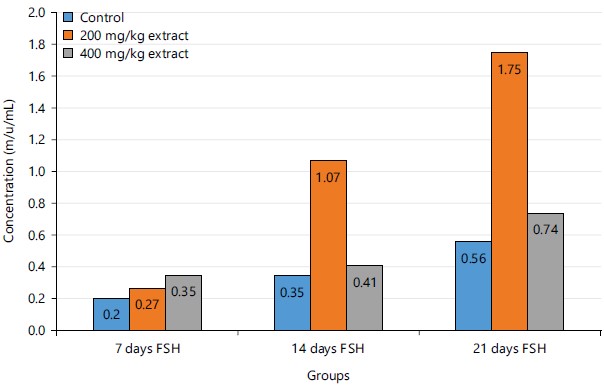
Figure 1 shows the concentration of follicle-stimulating hormone in experimental rats after 7, 14 and 21 days of administration of the extract (Cyperus esculentus, Phoenix dactylifera and Cocos nucifera). There was a significant increase in the control group, 200 mg/kg STCD group and 400 mg/kg STCD group, after 14 and 21 day.
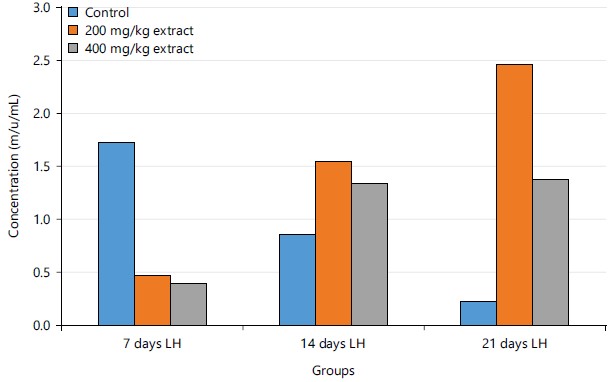
Figure 2 shows the concentrations of luteinizing hormone in experimental rats after 7, 14 and 21 days of administration of STCD. There was a significant decrease in the control group after 14 and 21 days, but there was a significant increase in both 200 mg/kg STCD group and 400 mg/kg STCD group, after 14 and 21 days.
| Table 1: | Percentage mean body weight difference in experimental rat model exposed to a synergistic mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera (STCD) | |||
| Groups | Initial body weight (g) |
Final body weight (g) |
Mean body weight difference (%) |
Organ weight (testes) (g) |
| Group 1 (control) | 69.80±7.50 |
121.00±30.83 |
30.95 |
1.42±0.73 |
| Group 2 (200 mg/kg STCD) | 88.20±5.12 |
121.20±29.20 |
8.39 |
2.44±1.09 |
| Group 3 (400 mg/kg STCD) | 111.80±6.72 |
123.00±11.68 |
2.86 |
4.14±0.59 |
| Values are expressed as Mean±Standard Deviation | ||||
|
|
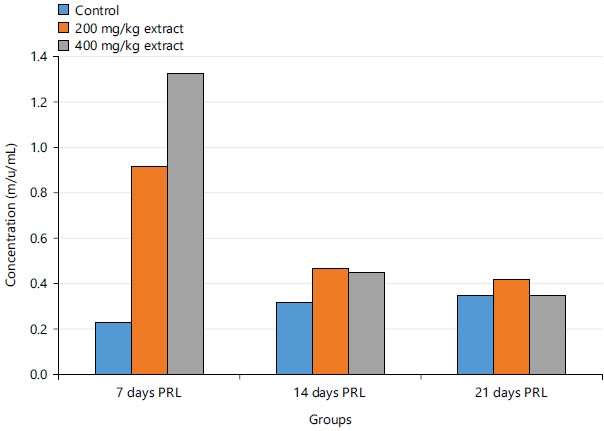
Figure 3 shows the concentrations of prolactin in experimental rats after 7, 14 and 21 days of administration of STCD. There was an increase in the control group, but there was a significant decrease in the 200 mg/kg STCD group and 400 mg/kg STCD group after 14 and 21 days.
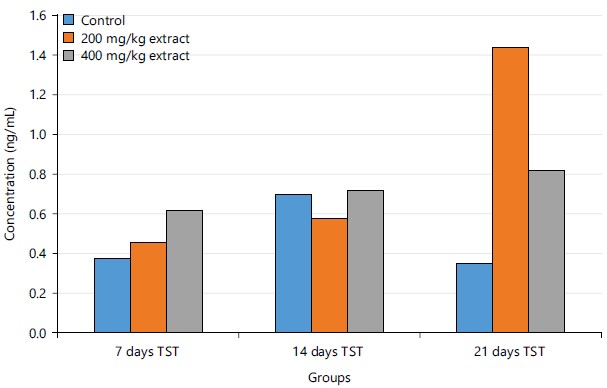
Figure 4 shows the concentrations of testosterone in the experimental rats after 7, 14 and 21 days of administration of STCD. The result shows that there was an increase in the control group after 14 days and a decrease after 21 days. However, there was a significant increase in the 200 mg/kg STCD group and 400 mg/kg STCD group after 14 and 21 days.
Table 2 shows the synergistic ingestion of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on male reproductive hormone activity. It showed that there was an increase in the concentration of follicle-stimulating hormone, luteinizing hormone and testosterone in the groups administered with 200 and 400 mg/kg STCD compared to the control group. However, there was a decrease in prolactin in the groups administered with 200 and 400 mg/kg STCD compared to the control group.
|
|
| Table 2: | Effect of synergistic ingestion of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera on male reproductive hormone activity in male rat model | |||
| Groups | FSH (m/u/mL) |
LH (m/u/mL) |
PRL (ng/mL) |
TST (ng/mL) |
| Group 1 (control) | 0.402±0.154a |
1.086±0.644a |
0.584±0.421a |
0.718±0.983a |
| Group 2 (200 mg/kg STCD) | 0.526±0.193a |
1.166±0.440a |
0.536±0.217a |
0.756±0.130a |
| Group 3 (400 mg/kg STCD) | 1.018±0.741a |
1.700±0.835a |
0.284±0.627a |
0.874±0.516a |
| Values are expressed as Mean±Standard Deviation, values with different superscripts are statistically significant at p≤0.05 and values with the same superscript are not statistically significant at p≤0.05 | ||||
|
|
|
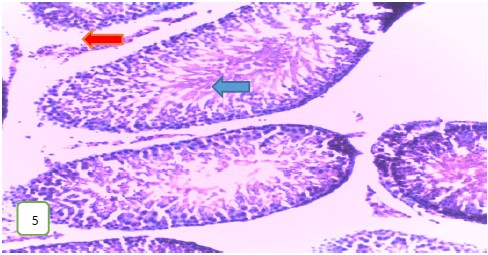
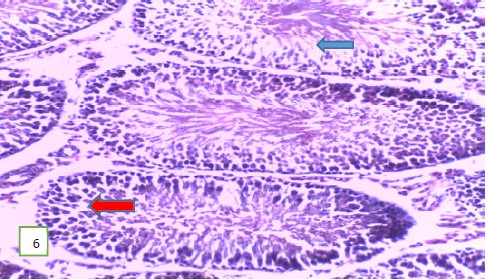
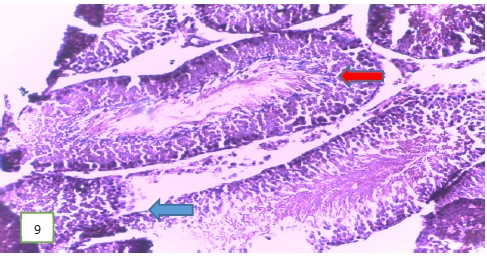
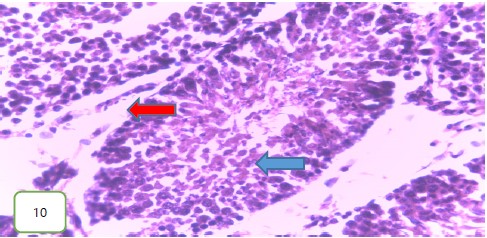
Histological examination of the testes of the experimental rats: The histological examination of the testes of the experimental rats was carried out. The results obtained showed that Fig. 5-7 which are the tissues for the Group 1 (control) experimental rats had normal seminiferous tubules and spermatozoa. Group 2 (200 mg/kg) (Fig. 8-10) experimental rats showed testes with normal seminiferous tubules containing numerous germ cells and normal spermatozoa at the center of the tubules.
|
|
|
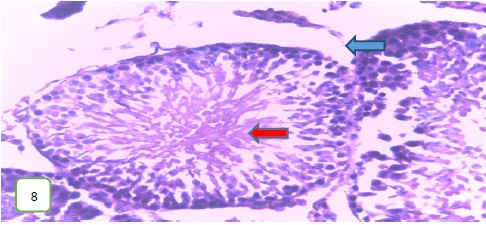
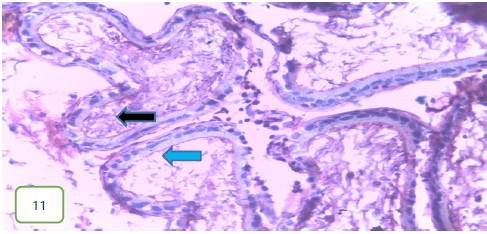
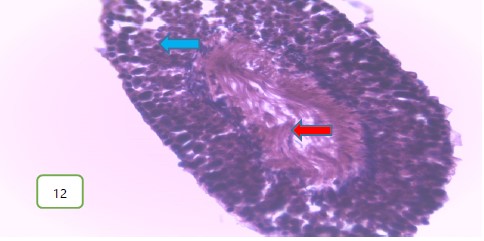
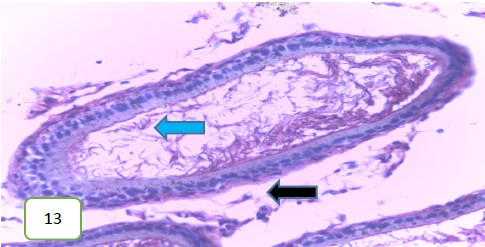
The testes of the experimental rats for group 3 (400 mg/kg) (Fig. 11 and 12) revealed a normal spermatic cord containing mature spermatozoa, while Fig. 13 revealed a normal epithelial lining and lumen containing mature sperm cells.
DISCUSSION
The synergistic mixture of Phoenix dactylifera, Cocos nucifera nuts and Cyperus esculentus (STCD) from this study showed no mortality of the male albino rats at all doses administered as reported in the previous publication of the project by Odinga et al.25. However, 3400 and 6800 mg/kg STCD led to diarrhea in the rats and 50% mortality was observed. The rat feed is composed of a nutrient content; of 18.50% protein, 3% fats, 6% crude fibers, 7% crude ash and 12% moisture. Animals allowed to unhampered exposure to high nutritional content food, water and standard conditions have recorded an immense body weight increase in experimental rats33.
|
|
|
Albeit the increase in the body weight of the rats administered 200 and 400 mg/kg STCD, a decrease in the percentage mean body weight difference was however observed in the groups administered 200 and 400 mg/kg extract when compared to the control group. The oral gavage method of administration of the extract to experimental rats has been reported by Turner et al.34 to have undulate effects on experimental rats which could lead to stress, result in passive reflux if the stomach is overfilled, aspiration pneumonia, pharyngeal, esophageal and gastric irritation or injury with structure formation.
From the above literature, the decrease in the percentage body difference in the extract-administered group could be attributed to the accompanying stress in the administration of the extracts. The result in Table 1 shows that there was an increase in the body weight of the animals after administration of the extract when compared to the initial body weight. The percentage mean difference in the body weight after administration of the synergistic mixture (Cyperus esculentus, Phoenix dactylifera and Cocos nucifera) was seen to be, for control 30.95 g, for 200 mg/kg STCD 8.39 g and 400 mg/kg STCD 2.86 g35. It could be seen that the testes for the control group had the lowest weight, while those in group 3 (400 mg/kg STCD) had the highest testicular weight. This is because the animals in group 3 had the highest weight; as such their testes had the highest weight. For control 1.42 g, for 200 mg/kg STCD 2.44 g and 400 mg/kg STCD 4.14 g in increasing order. Nwakanma et al.36 in their study reported protective effects of tiger nut on testicular and spermatogenesis process in hyperglycemic rats.
Figure 1 showed that there was an increase in the concentration of FSH in the control group after 14 and 21 days when compared to 7 days. From the results in Table 2, it was observed that there was an increase in the follicle-stimulating hormone of the groups administered with 200 and 400 mg/kg STCD when compared to the control but the increase was not statistically significant at 95% confidence interval. Follicle-Stimulating Hormone (FSH) in males has been reported to be involved in Sertoli cell proliferation. FSH correlates strongly with Sertoli cell number and testis size in adulthood37,38. This is of high importance, as the Sertoli cell number determines the quantity of sperm produced, a Sertoli cell is able to support a certain maximum number of germ cells39,40. Also for 200 and 400 mg/kg STCD, an increase after 14 and 21 days when compared to 7 days.
Figure 2 showed that there was a decrease in the concentration of LH in the control group after 14 and 21 days when compared to 7 days. However, there was an increase in both 200 and 400 mg/kg STCD after 14 and 21 days compared to 7 days. Male fertility is associated with the activities of LH. The LH is a precursor of testosterone, which functions in sperm production, motility and energy acquisition by way of augmenting sperm fructolysis and adenyl cyclase activity41. From the results in Table 2, there was an increase in the LH of the groups administered with 200 and 400 mg/kg STCD when compared to the control group, although not significant at p-value of 0.05. An increase in LH causes or triggers the testicles to make testosterone, which is important for producing sperm.
Figure 3 showed a simultaneous decrease in the concentration of Prolactin in both the control group, 200 and 400 mg/kg STCD after 14 and 21 days when compared to 7 days. Prolactin hormone (PRL), a hormone, synthesized in the adenohypophyseal lactotrophs, has no known target organ or defined role in males. Although the functional significance of prolactin to male reproduction has not been unequivocally established, the hormone has been associated primarily with male infertility42. From the results in Table 2, there was an insignificant statistical decrease in the activity of PRL when comparing control group to 200 and 400 mg/kg STCD.
Figure 4 showed an increase in the concentration of testosterone in the control group after 14 and a decrease after 21 days when compared to 7 days. However, a consistent increase in both 200 and 400 mg/kg STCD after 14 and 21 days when compared to 7 days was observed. Testosterone (TST), Table 2 show that there was an increase in the level of testosterone in the 200 and 400 mg/kg STCD when compared to the control group. Testosterone is responsible for the development of primary sexual development, which includes testicular descent, spermatogenesis, enlargement of the penis and testes and increasing libido43. It also plays an important role in the body, by regulating bone mass, fat distribution, muscle mass and strength and the production of red blood cells and sperm44. An increased testosterone enhances testicular size, libido and muscle mass.
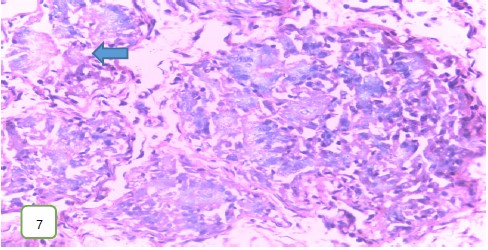
Results from histopathological examination photomicrographs revealed that the control group (Fig. 5-7) had normal seminiferous tubules containing numerous germ cells, with normal spermatozoa at the center of the tubules and massive necrosis which could be a result of exposure to infections while in the animal house45. For group 2 as seen in Fig. 8-10, (200 mg/kg STCD), the photomicrographs showed normal seminiferous tubules containing numerous germ cells, with normal spermatozoa at the center of the tubules. Figure 11-13 had photomicrographs for 400 mg/kg STCD experimental group. The photomicrographs revealed normal spermatic cords containing mature spermatozoa and also normal epithelial lining and lumen containing mature sperm cells were observed in all examined tissues. It has been proven that treatment with tiger nut extract improves sperm count and motility in male rats, which is associated with increased gonadotropins and testosterone serum levels46.
The findings of this study, as evidenced by the results obtained, imply that the STCD enhanced the activities of the male reproductive hormones FSH, LH, testosterone and prolactin, thus could be recommended for daily intake, dose-dependent. However, this study was limited to the acute investigation of the activities of the male reproductive hormones.
CONCLUSION AND RECOMMENDATIONS
The synergistic ingestion of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera (STCD) at 200 g/kg and 400 mg/kg, 24 hourly improved and increased the activity of follicle stimulating hormone, luteinizing hormone, prolactin and testosterone. This administration of STCD can be effective in enhancing male sexual hormones. The STCD could be taken regularly as it has no seemingly adverse effect at the right dose in the rat models but helped in boosting male sexual hormones. This study has proven that the speculation of these plant extracts to increase male sexual hormones is true.
This study therefore recommends the synergistic extract of Phoenix dactylifera, Cyperus esculentus and Cocos nucifera as a valuable natural and healthy drink for an enhanced aphrodiasaic and reproductive function in male rat models at a moderate dose. A trial in humans is also recommended. Further studies should be done on the long-term effects of the synergistic mixture of Cyperus esculentus (tiger nuts), Phoenix dactylifera (dates) and Cocos nucifera (coconut). Further studies should also be carried out on the mechanistic effects of these plant extracts.
SIGNIFICANCE STATEMENT
The global rise in rates of sexual dysfunctionality in males has gained much interest in the world of science, hence, a need for ways to enhance and manage sexual dysfunctionality. This study explored and proved the health benefits of Phoenix dactylifera, Cyperus esculentus and Cocos nucifera (STCD) extract on the male reproductive hormones. It further supports the speculated aphrodisiac activity of the STCD in male rat models.
ACKNOWLEDGMENTS
The authors acknowledge the Team Tigernut Students for their involvement in the Experimental process of this study: Ololube Chibuzor Christopher, Emilia Eric Allison, Esther Confidence Amadi, Richard Komene, Jackson Believe Zandra, Patience Nwinee, Cookey Queen, Joy Emenike and Enwukwe Favour Adaeze.
REFERENCES
- Odinga, T. and C.O. Nwaokezi, 2020. Effect of Ricinodendron heudelotii seed extract on the oxidative stress biomarkers of diabetic albino rats. J. Pharm. Res. Rev., 4.
- Ilahi, S. and T.B. Ilahi, 2022. Anatomy, Adenohypophysis (Pars Anterior, Anterior Pituitary). StatPearls Publishing, Treasure Island.
- Kotta, S., S.H. Ansari and J. Ali, 2013. Exploring scientifically proven herbal aphrodisiacs. Pharm. Rev., 7: 1-10.
- Tiwana, M.S. and S.W. Leslie, 2023. Anatomy, Abdomen and Pelvis: Testes. StatPearls Publishing, Treasure Island.
- Basaria, S., 2013. Reproductive aging in men. Endocrinol. Metab. Clin. North Am., 42: 255-270.
- Nassar, G.N. and S.W. Leslie, 2023. Physiology, Testosterone. StatPearls Publishing, Treasure Island.
- Rastrelli, G., G. Corona, E. Mannucci and M. Maggi, 2014. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: A meta-analytic study. Andrology, 2: 794-808.
- Rawindraraj, A.D., H. Basit and I. Jialal, 2023. Physiology, Anterior Pituitary. StatPearls Publishing, Treasure Island.
- Freeman, M.E., B. Kanyicska, A. Lerant and G. Nagy, 2000. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev., 80: 1523-1631.
- Hair, W.M., O. Gubbay, H.N. Jabbour and G.A. Lincoln, 2002. Prolactin receptor expression in human testis and accessory tissues: Localization and function. Mol. Hum. Reprod., 8: 606-611.
- Tunde-Akintunde, T.Y. and M.O. Oke, 2012. Thin-layer drying characteristics of tiger nut (Cyperus esculentus) seeds. J. Food Process. Preserv., 36: 457-464.
- Adejuyitan, J.A., E.T. Otunola, E.A. Akande, I.F. Bolarinwa and F.M. Oladokun, 2009. Some physicochemical properties of flour obtained from fermentation of tigernut (Cyperus esculentus) sourced from a market in Ogbomoso, Nigeria. Afr. J. Food Sci., 3: 51-55.
- Ogunlade, I., A.A. Bilikis and A.G. Olanrewaju, 2015. Chemical compositions, antioxidant capacity of tigernut (Cyperus esculentus) and potential health benefits. Eur. Sci. J., 11: 217-224.
- Gregg, C., V. Shikar, P. Larsen, G. Mak, A. Chojnacki, V.W. Yong and S. Weiss, 2007. White matter plasticity and enhanced remyelination in the maternal CNS. J. Neurosci., 27: 1812-1823.
- Allouh, M.Z., H.M. Daradka and J.H. Abu Ghaida, 2015. Influence of Cyperus esculentus tubers (tiger nut) on male rat copulatory behavior. BMC Complementary Altern. Med., 15.
- Sarraf, M., M. Jemni, I. Kahramanoğlu, F. Artés and S. Shahkoomahally et al., 2021. Commercial techniques for preserving date palm (Phoenix dactylifera) fruit quality and safety: A review. Saudi J. Biol. Sci., 28: 4408-4420.
- Al-Farsi, M.A. and C.Y. Lee, 2008. Nutritional and functional properties of dates: A review. Crit. Rev. Food Sci. Nutr., 48: 877-887.
- Ali-Mohamed, A.Y. and A.S.H. Khamis, 2004. Mineral ion content of the seeds of six cultivars of Bahraini date palm (Phoenix dactylifera). J. Agric. Food Chem., 52: 6522-6525.
- Maqsood, S., O. Adiamo, M. Ahmad and P. Mudgil, 2020. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem., 308.
- Rahmani, A.H., S.M. Aly, H. Ali, A.Y. Babiker and S. Srikar, 2014. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med., 7: 483-491.
- Gandhi, M., M. Aggarwal, S. Puri and S.K. Singla, 2013. Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male Wistar rat. Int. Braz. J. Urol., 39: 108-117.
- Odinga, T., B.R. Toghi, S.N. Golden, I. Austin-Aso and B.C. Lemii, 2023. Comparative assessment on the ameliorative potency of palm kernel oil and coconut water on castor oil-induced diarrhea. J. Pharmacol. Toxicol., 18: 76-82.
- Zulaikhah, S.T., J. Wahyuwibowo, R.D. Aziz, R.P. Dede and F.N. Ahmad, 2019. Effect of tender coconut water to prevent anemia on Wistar rats induced by lead (Plumbum). Pharmacogn. J., 11: 1325-1330.
- Airaodion, A.I., J.A. Ekenjoku, A.U. Megwas, K.O. Ngwogu and A.C. Ngwogu, 2019. Antihypertensive potential of coconut (Cocos nucifera L.) water in Wistar rats. Asian J. Res. Cardiovasc. Dis., 1: 8-15.
- Odinga, T.B., C.B. Lemii, I.R. Daka, C.U. Gabriel-Brisibe, S.K. Enebeli, I. Austin-Asomeji and F.U. Edward, 2023. Synergistic mixture of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera aqueous extract: Its liver and kidney benefits in male albino rat model. J. Biosci. Med., 11: 63-75.
- Bouhlali, E.D.T., A. Hmidani, B. Bourkhis, Z. Moussafir, Y. Filali-Zegzouti and C. Alem, 2023. Date (Phoenix dactylifera L.) fruits as a potential lipid-lowering therapy: Effect on high-fat diet and triton-WR-1339-induced hyperlipidemic rats. Drugs Drug Candidates, 2: 422-432.
- Botham, P.A., 2002. Acute systemic toxicity. ILAR J., 43: S27-S30.
- Lorke, D., 1983. A new approach to practical acute toxicity testing. Arch. Toxicol., 54: 275-287.
- Adefisayo, M.A., R.O. Akomolafe, O.S. Akinsomisoye, Q.K. Alabi, L. Ogundipe, J.G. Omole and K.P. Olamilosoye, 2018. Protective effects of methanol extract of Vernonia amygdalina (Del.) leaf on aspirin-induced gastric ulceration and oxidative mucosal damage in a rat model of gastric injury. Dose-Response, 16.
- Odinga, T.B., N. Nwachoko and C.U. Gabriel-Brisibe, 2020. Augmentation potency of ethanol seed extract of Ricinodendron heudelotii on some male reproductive hormones in Wistar albino rats. Int. J. Pharm. Chem., 6: 11-15.
- Odinga, T.B., E.O. Anyalebechi, N. Nwachoko, C.U. Gabriel-Brisibe, T.I. Aleruchi-Didia and C. Okwuonu, 2021. Ruinous effect on male reproductive hormones of Wistar albino rats exposed to air freshener. Asian J. Res. Rep. Endocrinol., 4: 129-134.
- Odukanmi, O.A., Q.B. Olusegun and S.B. Olaleye, 2019. Intestinal handling of glucose in Buccholzia coriacea treated male Wistar rats. J. Biosci. Med., 7: 87-98.
- Ranea-Robles, P., J. Lund and C. Clemmensen, 2022. The physiology of experimental overfeeding in animals. Mol. Metab., 64.
- Turner, P.V., T. Brabb, C. Pekow and M.A. Vasbinder, 2011. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci., 50: 600-613.
- Sanchez-Zapata, E., E. Fuentes-Zaragoza, J. Fernandez-Lopez, E. Sendra, E. Sayas, C. Navarro and J.A. Perez-Alvarez, 2009. Preparation of dietary fiber powder from tiger nuts (Cyperus esculentus) milk (Horchata) by-products and its physicochemical properties. J. Agric. Food Chem., 57: 7719-7725.
- Nwakanma, A.A., M.B. Ekong, I.C. Ngwuben, C.A. Idaguko and C.O. Elemuo, 2022. Cyperus esculentus L. protects testis and sperm morphology of hyperglycaemic rats. Int. J. Med. Surg. Sci., 9: 1-16.
- Allan, C.M., A. Garcia, J. Spaliviero, F.P. Zhang, M. Jimenez, I. Huhtaniemi and D.J. Handelsman, 2004. Complete sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: Evidence from genetic models of isolated FSH action. Endocrinology, 145: 1587-1593.
- Sharpe, R.M., C. McKinnell, C. Kivlin and J.S. Fisher, 2003. Proliferation and functional maturation of sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction, 125: 769-784.
- Abel, M.H., P.J. Baker, H.M. Charlton, A. Monteiro and G. Verhoeven et al., 2008. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology, 149: 3279-3285.
- Wreford, N.G., T.R. Kumar, M.M. Matzuk and D.M. de Kretser, 2001. Analysis of the testicular phenotype of the follicle-stimulating hormone β-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology, 142: 2916-2920.
- Ramanujam, L.N., W.X. Liao, A.C. Roy and S.C. Ng, 2000. Association of molecular variants of luteinizing hormone with male infertility. Hum. Reprod., 15: 925-928.
- Grattan, D.R., 2001. The Actions of Prolactin in the Brain During Pregnancy and Lactation. In: Progress in Brain Research, Russell, J.A., A.J. Douglas, R.J. Windle and C.D. Ingram (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-444-50548-4, pp: 153-171.
- Kalfa, N., L. Gaspari, M. Ollivier, P. Philibert, A. Bergougnoux, F. Paris and C. Sultan, 2019. Molecular genetics of hypospadias and cryptorchidism recent developments. Clin. Genet., 95: 122-131.
- Finkelstein, J.S., H. Lee, S.A.M. Burnett-Bowie, J.C. Pallais and E.W. Yu et al., 2013. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med., 369: 1011-1022.
- Krieger, J.N., U. Dobrindt, D.E. Riley and E. Oswald, 2011. Acute Escherichia coli prostatitis in previously health young men: Bacterial virulence factors, antimicrobial resistance and clinical outcomes. Urology, 77: 1420-1425.
- Gbotolorun, S.C., A.A. Salako and B. Ogunlade, 2022. Tiger nut: Antidote for alcohol-induced testicular toxicity in male sprague-dawley rats. JBRA Assist. Reprod., 26: 222-231.
How to Cite this paper?
APA-7 Style
Daka,
I.R., Odinga,
T., Lemii,
B.C., Gabriel-Brisibe,
C.U., Enebeli,
S.K., Austin-Asomeji,
I., Edward,
F.U. (2024). Male Reproductive Hormonal Activity of Synergistic Ingestion of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera in Male Rat Model. Asian Science Bulletin, 2(1), 60-73. https://doi.org/10.3923/asb.2024.60.73
ACS Style
Daka,
I.R.; Odinga,
T.; Lemii,
B.C.; Gabriel-Brisibe,
C.U.; Enebeli,
S.K.; Austin-Asomeji,
I.; Edward,
F.U. Male Reproductive Hormonal Activity of Synergistic Ingestion of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera in Male Rat Model. Asian Sci. Bul 2024, 2, 60-73. https://doi.org/10.3923/asb.2024.60.73
AMA Style
Daka
IR, Odinga
T, Lemii
BC, Gabriel-Brisibe
CU, Enebeli
SK, Austin-Asomeji
I, Edward
FU. Male Reproductive Hormonal Activity of Synergistic Ingestion of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera in Male Rat Model. Asian Science Bulletin. 2024; 2(1): 60-73. https://doi.org/10.3923/asb.2024.60.73
Chicago/Turabian Style
Daka, Iyaeneomi, Ransome, Tamuno-Boma Odinga, Barizoge Cletus Lemii, Christine Umanu Gabriel-Brisibe, Sarah Kelechi Enebeli, Iyingiala Austin-Asomeji, and Felicia Ucheawaji Edward.
2024. "Male Reproductive Hormonal Activity of Synergistic Ingestion of Aqueous Extract of Cyperus esculentus, Phoenix dactylifera and Cocos nucifera in Male Rat Model" Asian Science Bulletin 2, no. 1: 60-73. https://doi.org/10.3923/asb.2024.60.73

This work is licensed under a Creative Commons Attribution 4.0 International License.




