Microbial Products Mediated Stable Foam Formation under Controlled Environmental Conditions for Pollutant Management
| Received 07 Nov, 2023 |
Accepted 03 Apr, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Marine environment pollution is a significant threat to aquatic life. Addressing the removal or degradation of pollutants presents a substantial challenge for the scientific community. Bio-removal of contaminants using microbes and their products is an effective solution. Hence, the current study aimed to isolate and identify the marine microbial species for the production of stable foams and separation, characterization and application studies on stable foams. Materials and Methods: Marine sludge is the source for the isolation of organisms and the growth media used is Zobell marine broth. Isolated marine bacteria inoculated in Zobell marine broth and incubated at different environmental conditions. Physical parameters such as media composition, pH, temperature, agitation speed and flask type are optimized for stable foam formation. The obtained foam was subjected to various instrumental analyses to explore the constituents and the nature of the stable foam. Results: All the experimental studies prove that the marine bacterial isolate can able to form foam when grown in Zobell medium at 200 rpm, at pH 7.5 and temperature 30-37°C. The characterization studies reveal protein and other aliphatic molecules are the major constituents of the stable foam. Conclusion: The results of the study suggested the necessity of site-directed mutagenesis to understand the pathway of foam production in microorganisms. Further, the application study suggested the isolate might find immense application in bioremediation of heavy metals and other xenobiotics in soil and water and demand intensive research.
INTRODUCTION
Foams are abundant in nature. They universally occur in streams, rivers, lakes and sea/ocean waters. Natural foam is typically associated with colloids, carbohydrates, lipids and proteins initiated from the aquatic atmosphere1,2. Unnatural foam is associated with phosphates from farm fertilizers1 and detergents (organic and inorganic) discharged from pollution sources, especially from the paper and leather industry1,3-5. Generally, a combination of various constituents and environmental conditions is responsible for foam formation and its stability6-9.
Foams are nothing but an accumulation of small bubbles formed on or in a liquid by agitation or fermentation. A pure liquid cannot form foam unless or otherwise with the contribution of amphiphilic molecules. An ideal foam would be formed from the aqueous solution comprised of some major components such as hydrophilic-hydrophobic components, protein, fatty acids and some mineral salts under defined environmental conditions. The stability of foam is an important constraint in many applications. Current research is an additional concern about the development of the identical stable foam and its mechanism. Researchers used some adverse chemical substances to stabilize the foam's ability of the foam7,10,11. Still, there is a nonexistence of simple techniques to exhibit high stable foam ability and the aging of the foam12.
Hydrophobic particles allow the formation of long-lived foam by forming a solid layer in the interface. Hydrophilic particles will limit the liquid drain from the foam. However, amino acid-based surfactants can have a high influence on the interface bonding between the self-assemblies of the foaming particles that stabilize the foam13,14. Protein acts as macromolecular surface-active agents and is adsorbed in an air-water interface which impacts the dispersed system to stabilize foam15. Mineral salts are also good enhancers in the self-assembly process during foam formation. Moreover, with all these complementary features a stable foam can occur only with a minor amendment of peripheral trigger12. Environmental conditions (pH, temperature, light, aeration and agitation) also play a major role in the formation of stable foam8,9,12,14,16.
With prevailing reports on foam formation, several constituents play a major role and still, the mechanism behind is indistinct. The present study research group has already explored the biosurfactant-producing microorganisms that can able to form stable emulsions with enhanced self-assembly behavior17-23. With all this understanding, the current study proposed to explore the mechanism behind the foam formation and the role of constituents involved in the foam formation.
MATERIALS AND METHODS
Study area: The entire study has been conducted in the Microbiology division, CSIR-Central Leather Research Institute (CLRI), Chennai, India. The process of identification, optimization and characterization spanned a period of more than a year starting from November, 2011 to August, 2012.
Screening and culture conditions: Isolation, identification and characterization of the biosurfactant-producing organisms and their self-assembly properties were well explored in previous studies17-23. Zobell marine broth/agar media is used for the isolation of microbial species according to the standard procedure employed for the cultivation and maintenance of marine organisms. Microscopical, biochemical and physiological characterization of the marine isolate was executed to identify the genus level according to the standard procedures. All the pure cultures are stored at -80°C in 30% of glycerol. The obtained biosurfactant-producing pure cultures were cultured individually in Zobell marine broth at 37°C for 24-72 hrs under shaking conditions for the screening of foam formation. Followed by incubation, isolates exhibiting foam were subjected to 16S rDNA sequence analysis. The resulting sequences were aligned and analyzed with homologous sequences from other bacteria using the NCBI database entries.
Optimization of growth, nutrients and environmental conditions for foam formation: Followed by screening and identification, optimization of nutritional factors such as carbon sources (1-10%) and nitrogen sources (1-10%) in various concentrations were carried out. Further, different growth mediums (nutrient broth, Zobell marine broth and mineral medium) were also optimized for maximum formation of foam. Optimization of various culture conditions such as medium volume (5-25 % of flask volume), flask volume (100-5000 mL), flask type (Erlenmeyer and Haffkine), shaking speed (50-250 rpm), pH (4-8), temperature (25-40°C) and incubation period (24-120 hrs) were carried out for maximum foam formation. Following optimization, marine isolate was inoculated and incubated under optimized conditions.
Observation during growth period under optimized conditions
Growth and pH analysis: Growth profile and biomass production were assessed as per the standard protocol followed elsewhere. In brief, growth analysis was measured by turbidity measurement at 600 nm and 30 min intervals using UV-visible spectrophotometer (UV-2450, Shimadzu, Japan) and wet biomass was calculated after centrifugation of the culture broth at 10,000 rom at 4°C for 10 min and pH of the broth measured at 30 min interval using Elico pH meter, model CL 54.
Surface tension measurements: Both qualitative and quantitative surface activity measurements were carried out for the test samples. Qualitative measurement was carried out as per the procedure (Drop- collapse test) described by Tugrul and Cansunar24 and quantitative surface activity measured using GBX-3S tensiometer (DM) at room temperature conditions.
Physical observation of culture broth during incubation: During growth of the marine organism under shaking conditions, tiny white particles floated in the culture broth were observed after 24 hrs of incubation. Upon shaking, aggregation of particles and foam formation was observed after 48 hrs of incubation. Thick stable foam was observed on the top of the broth after 72 hrs of incubation. The obtained foam was recovered by filter decanting the aqueous medium and the obtained foam was lyophilized and subjected to yield physical (nature, odour, colour, solubility and pH) and instrumental (UV, FTIR, TGA and DSC) analyses.
Characterization of foam: Nature, color, texture and solubility of the recovered foam were analyzed. Microscopic analysis of the foam made by using Nikon Biocular microscope during the growth and after the recovery. Various Instrumental analyses were performed to characterize the foam sample using UV-Visible spectrum (Shimadzu UV-2450), FT-IR (Perkin–Elmer Co., USA model), CHNS (Euro EA 3000 Instruments), thermogravimetric (TGA Q50 (V20.6 Build 31) and differential scanning calorimeter (DSC Q200 (V23.10 Build 79).
In vitro foam simulation experiments: To understand the role of various constituents involved in the foam formation, in vitro experiments were conducted. All the optimized constituents (surface-active agents, salts, minerals metal ions and protein) and environmental factors (pH, temperature, aeration and shaking) were provided appropriately and kept for foam formation.
Replication of experiments: Three replicates have been conducted for all the procedures with five trials. The data represented in the results is an average of the experiments observed from the individual procedure.
RESULTS
Screening and culture conditions: From the marine samples, 18 morphologically distinct colonies were taken for foam formation. All 18 isolates were inoculated in Zobell marine broth and incubated for 24 to 92 hrs. Among 18 isolates, only one isolate was able to produce foam after 72 hrs of incubation. The morphology of the obtained marine isolate has creamy white, slimy and smooth colonies. The isolate was gram-positive rods with spores and motile. Biochemical analysis reveals positive for methyl red test and catalase. With respect to enzymatic hydrolysis analysis, starch, gelatin and lipids were positive. From the morphological and biochemical characterization results, the obtained marine isolate was identified as Bacillus sp. The 16S ribosomal DNA analysis of the isolate showed 98-99% similarity with the genus of Bacillus and the test organism designated as Bacillus sp.
Optimization of growth, nutrients and environmental conditions for foam formation: To attain maximum foam formation, growth media, carbon source, nitrogen source, medium volume, flask type, shaking speed, pH, temperature and incubation period were optimized. With respect to optimization of growth medium, maximum foam formation was observed in Zobell marine medium, whereas in nutrient broth and mineral medium, only the growth was observed without foam formation. One percent of carbon and nitrogen sources was sufficient for the maximum formation of foam. With respect to the environmental factors, 10% of flask volume, 150 rpm for shaking speed, pH 7, temperature 37°C in Haffkine’s as well Erlenmeyer flask for 72 hrs of incubation period.
With respect to the pH effect on foam formation, observed that pH 5.0 inhibited the growth of marine isolate and the media was clear. At pH 7.7 tremendous growth of marine isolate with maximum foam formation and at pH 9 microbial growth without foam formation. With respect to effect on foam formation upon varying temperatures shows that at 28 and 37°C, the marine isolate can able to grow well and form foam but maximum foam formation was observed only at 37°C, whereas at 40°C only the growth of marine isolate was observed without any foam formation.
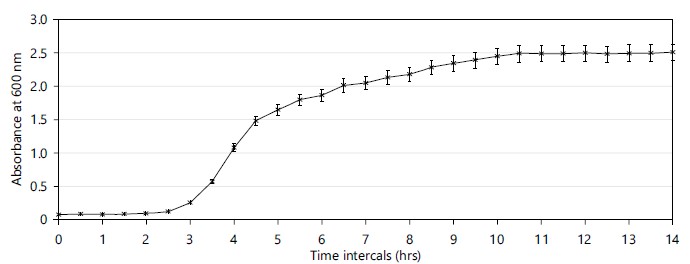
Observation during growth period under optimized conditions: Under optimized conditions, the growth profile of marine isolates at 30 min time intervals was observed. The marine isolate follows a typical growth pattern, lag, log and stationary phase achieved after 600 hrs of incubation and the doubling time of the isolate was calculated as 30 min (Fig. 1).
During the growth, the pH of the medium showed an increase and the final pH of the medium observed was >8.5±0.4. Surface activity measurements made during the growth of the marine isolates exhibit surfactant activity after 24 hrs of incubation and the maximum surfactant activity was observed after 48 hrs and maintained till 72 hrs. The maximum surface tension was observed as 35 mN/m after 72 hrs of incubation. Wet weight of cells obtained from 1000 mL of broth was 17.66 g. The dry weight of obtained cells was 5.44 g.
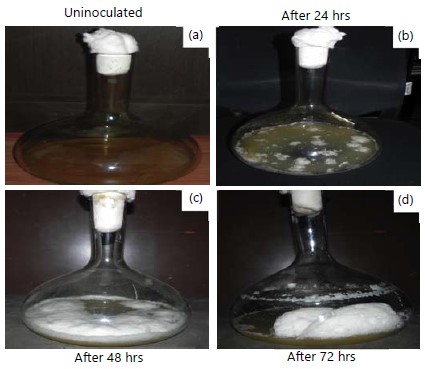
To ensure the contamination, an uninoculated control flask (Fig. 2a) is maintained throughout the entire incubation period. Under optimized conditions, the organism attained the maximum growth and mild froth was observed after 24 hrs (Fig. 2b). After 48 hrs of incubation, a white sponge-like foam was observed (Fig. 2c).
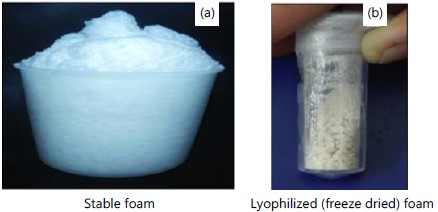
After 72 hrs of incubation, yield of the stable foam was increased (Fig. 2d) and the obtained foam (Fig. 3a) was lyophilized after recovery (Fig. 3b). Marine Bacillus sp., can able to produce 4.428 g lyophilized foam from 1 L of Zobell marine medium.
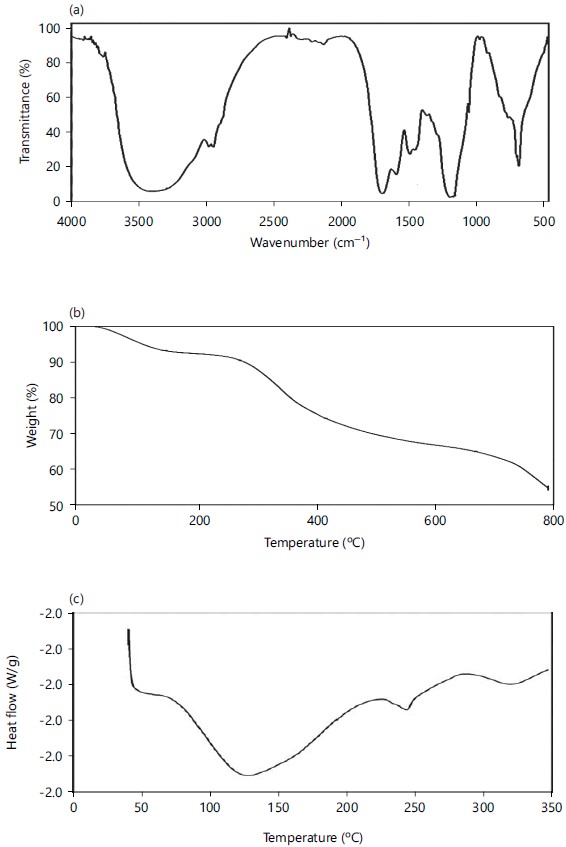
Instrumental characterization of foam: The obtained lyophilized foam was subjected to biochemical and instrumental analysis. The foam was partially soluble in water, ethanol, methanol, acetone and dimethyl formamide whereas foam solubility is comparatively better in dimethly sulfoxide and it has 30 μg/mL of protein content. With respect to the FTIR analysis, the lyophilized foam exhibits the presence of -OH-,-C-H, C-H, -C=O, N-H; C-N, C-N, -C-O and ≡CH linkages with the corresponding peaks at 3402.7 2964.86, 2926.9,1650.3, 1538.7, 1440.3, 1404.3, 1145. and 639.6 cm–1, respectively (Fig. 4a). The TGA results reveal, that the obtained foam exhibited stability till 275°C and at 800°C 54% of the foam sample was left as a residue (Fig. 4b) infers the high stability of the sample. The DSC analysis reveals, that the obtained foam sample shows glass transition Tg at 126.41°C and 242.84°C (Fig. 4c). The CHNS analysis of the foam reveals 14 to 19% of carbon, 2 to 3% of hydrogen and 4 to 5% of nitrogen and no sulphur content.
|
|
|
|
In vitro foam simulation experiments: Foam simulation experiments revealed that formation of foam was observed in the presence of all optimized ingredients and the environmental factors. However, the observed foam was very fragile and unstable. The quantity of the obtained foam was very low and it collapsed when the shaking interrupted. Figure 5 illustrates the hypothesis behind the stable foam formation, which is mediated by microbial products and environmental factors.

|
DISCUSSION
The present study reveals the role of microbial surface-active agents and other constituents (produced during the growth of the marine bacteria) on stable foam formation and its hypothesis. The marine isolate used in the present study produced a significant potential surface-active agent and facilitated stable foam formation. In addition, nutritional and environmental factors play a key role in stable foam formation. First and foremost, Zobell marine broth consists of salts and metal ions, carbon and nitrogen sources along with optimized pH, temperature can greatly influence microbial growth which enhances the synthesis of biosurfactants19.
After 24 hrs of incubation under optimized conditions, extreme production of biosurfactant reduces the surface tension (35 mN/m) in the growth media and reaches critical micelle concentration (CMC) which initiates foam formation under shaking conditions (150 rpm). Shaking under uniform conditions provides suitable aeration that induces lamellae/micelle formation. In addition, flask type (Erlenmeyer/Haffkine’s), flask size (500-5000 mL) and media volume (10% flask size) also improve the aeration during incubation, which ultimately provokes the foam formation. After 48 hrs of incubation, continuous production of biosurfactant, proteins and secondary metabolites of microbial species increases the viscosity to the growth media that helps in aggregation of lamellae/micelles. Accumulation of lamellae/micelles by absorbing onto the air bubbles forms macro foam structures and protects the air bubbles from burst25. The effect of biosurfactant on the formation and stability of foam is dependent on the surfactant type, particle size and concentration.
In general, foam formation in the aquatic environment is inevitable. Formation of foam might be a global problem but sometimes it can be used in remediation developments26 and as an indicator of a polluted environment27. Foam is nothing but a diffusion of gas in a solid or liquid separated by a flimsy liquid or lamellae-like thin layer. A liquid with surface active agents, metal/organic salts and carbon/nitrogen sources along gas bubbles can able to persuade foam formation28,29. Most of the foam formation has been induced by chemical components and is hazardous to the environment. Hence, biological products carry the research in a better way constantly in that secondary biological products from marine microorganisms are effective. Several reports revealed that foam formation occurs with an amalgamation of certain components and does not ensue in pure liquid. However, the mechanism behind the foam formation in the aqueous environment and the constituent responsible for stable foam formation is still challenging to disclose to the researchers. This study reveals the role of microbial surface-active agents and other constituents (produced by marine bacteria) on stable foam formation and its hypothesis.
Further, continuous incubation increases the interaction between the molecules present in the growth media. Gravity between the molecules (liquid and air bubbles) in the highly viscous liquid contains various constituents and is the driving force for stable foam6,28,29. Partially hydrophobic molecules enhance the affinity between the interfaces of self-assembled moieties and provide mechanical stability to the foam28. In the present study, microbial cells present in the growth medium act as hydrophobic molecules and stabilize the foam29. In addition, salts in the growth medium increase the surface active agents' critical packing parameter (CPP) that helps in the close-fitting of self-assembly at air-water surface and surface active proteins synthesized by microbial species also involved in the foam stability30.
The present study acknowledges the significant threat posed by pollution in marine environment to aquatic life, highlighting the urgency of finding effective solutions for pollutant removal or degradation. The study suggests that utilizing marine microorganisms and their products for bioremediation purposes could be a promising approach to address marine pollution. Isolating and identifying marine bacterial species capable of producing stable foam, adds to the scientific understanding of how microorganisms can be harnessed for remediation efforts. The potential future directions such as exploring the pathway of foam production and investigating various applications for targeting bioremediation or biodegradation of heavy metal and xenobiotics in marine environments realize new approaches to environment pollution. Moreover, the efficacy of the bioremediation may vary when compared to the controlled environment and needs to be investigated thoroughly, leaving opportunities for further research and validation.
CONCLUSION
The current research study explored the in situ self-assembly of microbial products such as biosurfactants, protein and other metabolites by marine microbial approach. Remarkably, the current investigation established the interaction between the microbial molecules with metal ions and salts present in the media can able to initiate foam formation. In addition, air-water interfacial tension is effectually controlled by other microbial proteins and components that enhance the foam to be more stable. Moreover, the mechanism behind the foam formation by marine microbes needs to be explored more to reveal the hypothesis behind current research.
SIGNIFICANCE STATEMENT
Industrial pollutants impact the health of both humans and soil. Various physical, chemical and biological processes have been adopted for the removal of pollutants. The generation of secondary pollutants is the major drawback in physical and chemical processes. The biological processes involve microorganisms, which are able to degrade or disintegrate the pollutants based on the concentration and the major drawbacks are the selection of effective microbial sources and the time consumption. However, few microbial products are able to remove the pollutants effectively through foam formation. The stable foam generated by the microbial products is able to carry the pollutants without disturbing the source sample. The present study details the stable foam formation by the microbial products of chosen microbes and its importance in the removal of pollutants.
ACKNOWLEDGMENT
One of the authors thanks the Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance in the form of Senior Research Fellowship (SRF) Award No: 31/6(347)/2011-EMR-I.
REFERENCES
- Ettema, R., J.K. Johnson and J.A. Schaefer, 1989. Foam-initiated ice covers on small rivers and streams: An observation. Cold Reg. Sci. Technol., 16: 95-99.
- Napolitano, G.E. and J.E. Richmond, 1995. Enrichment of biogenic lipids, hydrocarbons and PCBs in stream-surface foams. Environ. Toxicol. Chem., 14: 197-201.
- Madrange, L., P. Ehabouryi, O. Ferrandon, M. Mazeti and J. Rodeaud, 1993. Study of the formation and stability of chemical foam on the Vienne River between Limogesançi Confolens. J. Water Sci., 6: 315-335.
- Fisenko, A.I., 2004. A new long-term on site clean-up approach applied to non-point sources of pollution. Water Air Soil Pollut., 156: 1-27.
- Ruzicka, K., O. Gabriel, U. Bletterie, S. Winkler and M. Zessner, 2009. Cause and effect relationship between foam formation and treated wastewater effluents in a transboundary river. Phys. Chem. Earth Parts A/B/C, 34: 565-573.
- Wegner, C. and M. Hamburger, 2002. Occurrence of stable foam in the upper Rhine River caused by plant-derived surfactants. Environ. Sci. Technol., 36: 3250-3256.
- Salonen, A., M. In, J. Emile and A. Saint-Jalmes, 2010. Solutions of surfactant oligomers: A model system for tuning foam stability by the surfactant structure. Soft Matter, 6: 2271-2281.
- Fameau, A.L., S. Lam and O.D. Velev, 2013. Multi-stimuli responsive foams combining particles and self-assembling fatty acids. Chem. Sci., 4: 3874-3881.
- Fameau, A.L. and A. Salonen, 2014. Effect of particles and aggregated structures on the foam stability and aging. C. R. Phys., 15: 748-760.
- Cox, A.R., D.L. Aldred and A.B. Russell, 2009. Exceptional stability of food foams using class II hydrophobin HFBII. Food Hydrocolloids, 23: 366-376.
- Vignes-Adler, M. and D. Weaire, 2008. New foams: Fresh challenges and opportunities. Curr. Opin. Colloid Interface Sci., 13: 141-149.
- Fameau, A.L., A. Saint-Jalmes, F. Cousin, B.H. Houssou and B. Novales et al., 2011. Smart foams: Switching reversibly between ultrastable and unstable foams. Angew. Chem. Int. Edn., 50: 8264-8269.
- Takehara, M., H. Moriyuki, I. Yoshimura and R. Yoshida, 1972. Surface active N-acylglutamate: II. Physicochemical properties of long chain N-acylglutamic acids and their sodium salts. J. Am. Oil Chem. Soc., 49: 143-150.
- Zhang, D., Y. Sun, Q. Deng, X. Qi, H. Sun and Y. Li, 2016. Study of the environmental responsiveness of amino acid-based surfactant sodium lauroylglutamate and its foam characteristics. Colloids Surf. A: Physicochem. Eng. Aspects, 504: 384-392.
- Damodaran, S., 2005. Protein stabilization of emulsions and foams. J. Food Sci., 70: R54-R66.
- Salonen, A., D. Langevin and P. Perrin, 2010. Light and temperature bi-responsive emulsion foams. Soft Matter, 6: 5308-5311.
- Gnanamani, A., V. Kavitha, G. Sekaran and G.S. Rajakumar, 2008. Vesicle formation in hydrocarbons assisted with microbial hydrolases and biosurfactants. Colloids Surf. B: Biointerfaces, 67: 192-198.
- Gnanamani, A., V. Kavitha, N. Radhakrishnan, G. Sekaran, G.S. Rajakumar and A.B. Mandal, 2010. Microbial biosurfactants and hydrolytic enzymes mediates in situ development of stable supra-molecular assemblies in fatty acids released from triglycerides. Colloloids Surf. B: Biointerfaces, 78: 200-207.
- Gnanamani, A., V. Kavitha, N. Radhakrishnan and A.B. Mandal, 2010. Bioremediation of crude oil contamination using microbial surface-active agents: Isolation, production and characterization. J. Bioremed. Biodegrad., 1.
- Kavitha, V., A.B. Mandal and A. Gnanamani, 2015. Biotransformation of soybean oil to a self-healing biopolymer. Biocatal. Biotransform., 33: 29-37.
- Kavitha, V., N. Radhakrishnan, E. Madhavacharyulu, G. Sailakshmi and G. Sekaran et al., 2010. Biopolymer from microbial assisted in situ hydrolysis of triglycerides and dimerization of fatty acids. Bioresour. Technol., 101: 337-343.
- Kavitha, V. and A. Gnanamani, 2013. A multilayered supramolecular self-assembled structure from soybean oil by in situ polymerization and its applications. Indian J. Exp. Biol., 51: 400-405.
- Radhakrishnan, N., V. Kavitha, E. Madhavacharyulu, A. Gnanamani and A.B. Mandal, 2011. Isolation, production and characterization of bioemulsifiers of marine bacteria of coastal Tamil Nadu. Indian J. Geo-Mar. Sci., 40: 76-82.
- Tugrul, T. and E. Cansunar, 2005. Detecting surfactant-producing microorganisms by the drop-collapse test. World J. Microbiol. Biotechnol., 21: 851-853.
- Harden, S.L. and D.F. Williams, 1989. Stable carbon isotopic evidence for sources of particulate organic carbon found in sea foam. Estuaries, 12: 49-56.
- Vikingstad, A.K., M.G. Aarra and A. Skauge, 2006. Effect of surfactant structure on foam-oil interactions: Comparing fluorinated surfactant and alpha olefin sulfonate in static foam tests. Colloids Surf. A: Physicochem. Eng. Aspects, 279: 105-112.
- Wilson, M.I., L.D. Robertson, M. Daly and S.A. Walton, 1995. Effects of visual cues on assessment of water quality. J. Environ. Psychol., 15: 53-63.
- Pugh, R.J., 1996. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci., 64: 67-142.
- Heard, J., E. Harvey, B.B. Johnson, J.D. Wells and M.J. Angove, 2008. The effect of filamentous bacteria on foam production and stability. Colloids Surf. B: Biointerfaces, 63: 21-26.
- Eisenreich, S.J., A.W. Elzerman and D.E. Armstrong, 1978. Enrichment of micronutrients, heavy metals, and chlorinated hydrocarbons in wind-generated lake foam. Environ. Sci. Technol., 12: 413-417.
How to Cite this paper?
APA-7 Style
Varadharajan,
K., Arumugam,
G. (2024). Microbial Products Mediated Stable Foam Formation under Controlled Environmental Conditions for Pollutant Management. Asian Science Bulletin, 2(3), 280-289. https://doi.org/10.3923/asb.2024.280.289
ACS Style
Varadharajan,
K.; Arumugam,
G. Microbial Products Mediated Stable Foam Formation under Controlled Environmental Conditions for Pollutant Management. Asian Sci. Bul 2024, 2, 280-289. https://doi.org/10.3923/asb.2024.280.289
AMA Style
Varadharajan
K, Arumugam
G. Microbial Products Mediated Stable Foam Formation under Controlled Environmental Conditions for Pollutant Management. Asian Science Bulletin. 2024; 2(3): 280-289. https://doi.org/10.3923/asb.2024.280.289
Chicago/Turabian Style
Varadharajan, Kavitha, and Gnanamani Arumugam.
2024. "Microbial Products Mediated Stable Foam Formation under Controlled Environmental Conditions for Pollutant Management" Asian Science Bulletin 2, no. 3: 280-289. https://doi.org/10.3923/asb.2024.280.289

This work is licensed under a Creative Commons Attribution 4.0 International License.




