Analysis of HPV in Cervical Lesion/Cervical Cancer Using P16 by Immunohistochemical Method
| Received 14 Nov, 2023 |
Accepted 20 Apr, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Human Papillomavirus (HPV) is a group of viruses implicated in certain cancers worldwide. It is divided into low-risk and high-risk types. The HPV transmission is majorly by sexual transmission. This study involves the use of immunological markers in identifying the various stages of cancer progression, as well as in diagnosing cancer, predicting prognosis and potentially serving as therapeutic targets for squamous cell carcinoma and cervicitis. Therefore, this study aims to analyze Human Papillomavirus (HPV) using P16 by immunohistochemical method. Materials and Methods: The study population is a 10 year retrospective cross-sectional study using Formalin-Fixed Paraffin-Embedded (FFPE) archival cervical tissues from the Histopathology Laboratories in Federal Capital Territory, Gwarimpa General Hospital, Wuse General Hospital, Asokoro General Hospital, University of Abuja Teaching Hospital and Maitama General Hospital from 2005 to 2015. A total of 80 samples were selected and analyzed using Haematoxylin and Eosin staining techniques and avidin biotin complex (ABC) methods for histological classification and p16 immunostaining, respectively. Results: The P16 immunoreactivity revealed a statistically significant rate of positivity as was found in the p16 expression among cases studied. Cervicitis (CC) showed a positive expression of 11(55.0%), cervical intraepithelial neoplasm (CIN 1) showed a higher positive expression of p16, 13(86.7%) significant at (p<0.05). The CIN 2 showed a high rate of positive expression 9(60.0%) still significant. There was a significantly higher expression of p16 pattern of 22(73.3%) among the squamous cervical cells (SCC). Conclusion: The p16 immunoreactivity predicts high-risk HPV and relates very strongly with High-grade Squamous Intraepithelial Lesions (HSIL) and cervical cancer carcinogenesis including some cervicitis.
INTRODUCTION
Cervical cancer is the fourth most common cancer in women worldwide with about 570,000 new cases in 2018, which represents 6.6% of all cancer deaths in females, especially in developing countries of the world such as Nigeria. Based on estimates1, cervical cancer is the second most frequent malignancy among women in less developed nations; in 2018, accounting for 84% of all new cases. The results of post-marketing surveillance and clinical trials have demonstrated the high safety and efficacy of HPV vaccinations in preventing infections. If caught early enough in the disease, cervical cancer is curable1,2.
Cervical cancer is caused by HPV persistent infection and can be detected by koilocytotic diagnostic features using Haematoxylin and Eosin, PCR molecular method and immunohistochemical evaluation3,4. Seventy percent of cervical malignancies and precancerous cervical lesions are caused by two HPV types (16 and 18)3.
The cervical epithelium’s chronic high-risk HPV infection seems to be the catalyst for the development of cancer5. The cervical mucus’s viscosity and water content change throughout the menstrual cycle. The mucus has a stretchy quality known as Spinnbarkeit, which is especially noticeable during ovulation. In addition, the cervix has a crucial role in birthing6,7.
When the uterus starts to contract, the support that the cervix gives the fetal head starts to give way. To accommodate the bead on the fetus as it descends from the uterus to the vagina during birthing, the cervix must widen to a diameter of more than 10 cm (3.9 in)8. All things considered, p16 immunohistochemistry is used to evaluate cervical intraepithelial neoplasia linked to HPV. Nevertheless, it is important to remember that p16's diagnostic utility in HIV infection is unknown9.
A component of the female reproductive system is the cervix. The narrower lower portion of the uterus is connected above with the broader top half or body, of the uterus, measuring approximately 2-3 cm (0.8-1.2 in) in length10. The vaginal portion of the cervix, also known as the ectocervix, is the lower end of the cervix that protrudes through the vagina’s anterior wall, while the supravaginal portion of the cervix is the remaining portion of the cervix that is above the vagina11.
According to the previous study by Osol and Mandala12, the uterus’s internal orifice (sometimes called the external os) and exterior orifice are the names given to the apertures, respectively. The mucosa covering the ectocervix is referred to as the exocervix and the mucosa lining the cervical canal is known as the endocervix. According to Delnooz et al.13, the cervix has an inner mucosal layer, a thick layer of smooth muscle and a serosa covering made of connective tissue and the peritoneum that covers it posteriorly in the supravaginal area.
It is twice as large during childhood and eventually reaches its adult size after puberty when it is smaller than the uterus14. The paramesonephric duct gives rise to the original columnar epithelium, while the urogenital sinus gives rise to the original squamous epithelium of the cervix during fetal development. The initial squamocolumnar junction is the location where these two original epithelia converge14.
When cervical cancer reaches an advanced stage, some of the symptoms include irregular menstrual cycles or atypical vaginal bleeding after sexual activity; back, leg or pelvic pain; exhaustion, weight loss, or lack of appetite; odorous vaginal discharge and swollen legs in one foot. Consequently, it is clear from these data that efficient screening and treatment initiatives might significantly lower the high global death rate from cervical cancer, which is estimated to be 52%. The study aims to analyze Human Papillomavirus (HPV) using P16 by immunohistochemical method.
MATERIALS AND METHODS
Study duration: The study population was from 10 years of retrospective archival tissue blocks of cervical cancer among women in Abuja metropolis from June, 2005 to July, 2015.
Study area: The University of Abuja Teaching Hospital (UATH), Mataima General Hospital, Gwarinpa General Hospital and National Hospital in the Federal Capital Territory of Abuja were the medical facilities where the research was conducted. Additionally, the analysis will be conducted at the Research Centre India and the Cachar Cancer Hospital. Data was gathered to improve the research’s credible and successful outcomes.
Analytical site: Situated on property donated by the Assamese government, the Cachar Cancer Hospital and Research Centre is a comprehensive cancer care facility that is recognized by the DSIR SIRO (Govt. of India) and is not for profit. The Cachar Cancer Hospital Society, a non-profit organization, is in charge of its administration. Situated in the Barak Valley of Assam, India, on the outskirts of Silchar town, is the Cachar Cancer Hospital and Research Centre (CCHRC). It was founded in 1996 and is run by the Cachar Cancer Hospital Society, a non-profit organization registered under the Societies Registration Act.
Sample technique: Total of four study groups as well as a control group will be formed from the study samples. The individuals in the study group include control, cervicitis, CIN and squamous cell carcinoma patients.
The sample size will be determined using15:
Where:
n |
= | Sample size | |
| N | = | Population under study | |
| e | = | Margin error |
Sample collection: Using a microtome and microtome blade, sections of roughly 3 to 5 microns are cut from the chosen samples (blocks), which are then placed on IHC-specific slides and stored for IHC staining. Slides are baked at 60°C for an hour before staining to prevent wash-off during IHC staining.
Sample analysis: The IHC analysis for P16 using Dako the Immune reactivity target is in the cytoplasm.
Statistical analysis: Using Microsoft Access software, the Statistical Package for Social Science (SPSS Windows 22.0) and EPI information, the case and control findings were compiled and managed statically. The data analysis will be conducted using SPSS; all variables were analyzed using descriptive statistics to determine the associations between the independent variables in the study.
Summary of ethical issue involved in the research: The potential subject needs to be fully educated about the purpose, procedures, expected advantages, possible risks and discomfort that the study may cause. Every subject has a right that needs to be honored. Additionally, the participant will be made aware of their ability to withdraw from the study at any time. The subjects’ interests were prioritized over the needs of the research.
Consequences of the study for the local community, environment and participants: There were no negative effects on the participants, the local population or the environment because safety precautions were taken to guarantee that any waste produced by the medical facilities was properly disposed of.
Dissemination of results of study: The study’s outcomes were intended for academic purposes; yet, they will be utilized to identify the best course of action for cancer patient management, prevention, therapy and raising public awareness of cervical cancer-related issues.
Confidentiality and privacy: Following the gathering of the analysis’s findings, the consent forms containing the participants’ biodata must be promptly destroyed to protect privacy and confidentiality. Only the participants’ age, sex and sample type will be disclosed; the names of participants will not.
Inclusion criteria: The diagnosis of cervical tumors was confirmed by reviewing all slides stained with Hematoxylin and Eosin. Additionally, sufficient cancer tissue depiction was guaranteed for each and every tumor case that was chosen. The age range needs to start at 17 years old.
Exclusion criteria:
| • | Study excluded instances not classified as cervical malignancies or lesions, as well as inadequate tissue sections | |
| • | Cases for whom there was no clinical information in the medical records or for which there were damaged or missing tissue blocks were not included |
Procedure for handling and treatment of archived tissue blocks: From the archival storage area, the preserved tissue blocks that represented the cancer diagnosis were taken out. Appropriate choices were determined by assessing the tissue’s current condition, which includes examining the tissue’s orientation, physical tissue adequacy on the paraffin blocks and the existence or lack of molds and dust particles. The tissues that needed it underwent re-embedding.
A rotary microtome was used to segment all of the chosen samples at a thickness of three microns and the conventional Haematoxylin and Eosin staining technique was applied. After two pathologists examined the slides independently, they were diagnosed with squamous cell carcinoma, CIN 1, CIN 2 and cervicalitis.
To represent the four antibody markers utilized in this investigation, four sections of 3 microns were further sliced for each sample. Additionally, three-micron sections were cut to identify positive and negative controls for each of the four different antibodies.
After being deparaffinized in xylene, the tissue sections were hydrated with decreasing alcohol grades and then they were cleaned in water. Using a heat-mediated antigen retrieval technique that needed a pressure cooker and PH 6.0 citric acid, the antigenic sites of cancer cells were recovered. After being chilled in water, tissue pieces were prepared for the next steps of the immunohistochemistry procedure.
Principle of Haematoxylin and Eosin staining: The most popular stain in histology and histopathology labs for displaying a variety of normal and aberrant cells and tissue components is Hematoxylin and Eosin. While Eosin stains cell cytoplasm and the majority of connective tissue fibres in various hues and intensities of pink, orange and red, hematoxylin component stains the nuclei of cells blue-black, revealing good intra-nuclear features.
The basic component of cells that has an affinity for acidic dye and the acidic component of cells that has an affinity for basic dye form the basis of the principle. The nucleus is the acidic portion of the cell in Hematoxylin and Eosin stains. Because of this, hematoxylin is referred to as a nuclear stain, whereas Eosin functions as an acidic stain, binding to the cytoplasm, the fundamental component of the cell and giving a pink stain.
Immunohistochemistry method16: The technique is called the avidin biotin complex (ABC) method or the avidin biotin immunoperoxidase method. Formalin-fixed and paraffin-embedded tissue that was sliced microns thick was used for the IHC. Using a pressure cooker and a PH 6.0 citric acid solution, tissue antigenic sites were extracted. Hydrogen peroxide, avidin and biotin blocks were used for peroxidases, proteins and biotin, respectively. For the investigation, sections were treated with various antibodies. The haematoxylin counterstain, streptavidine, DAB/substrate reaction and biotylinated secondary antibody came next.
The antibody dilution factor used is 1:50 for p16.
Below is the detail of the IHC protocol: Sections of the processed tissue were brought down to water by passing them through two changes of xylene, three changes of lowering grades of alcohol and finally water. The tissue was sectioned at two microns using a rotary microtome and heated to 70°C for at least an hour. The sections were heated for 15 min at power 100 in a PH 6.0 citric acid solution to extract the antigen. To allow the portions to cool, the hot citric acid was gradually replaced by cool water over a minimum of five minutes. After washing the sections with PBS, they were incubated with the corresponding diluted primary antibody, such as E-cadherin antibody diluted 1:100, for 60 min.
One drop (20 microns) of the DAB chromogen and one millilitre of the DAB substrate are combined to create a functioning DAB solution. Sections are treated with this working solution after the HRP has been cleaned off with PBS for at least 5 min. This is the time when the brown reactions start to show themselves, especially for a positive objective. Water is used to rinse out any remaining DAB solution and precipitate. After at least two minutes of counterstaining with hematoxylin solution, sections were temporarily blued. Sections are cleaned in xylene, dehydrated in alcohol and mounted in DPX.
Depending on the antigenic sites, cells with particular brown hues in the cytoplasm, cell membrane or nuclei are regarded as positive.
Cells stained with hematoxylin and lacking any brown coloration receive a negative score. Brown artifacts and non-specific binding to connective tissue and cells are ignored.
Immunohistochemical methods16:
| • | IHC method was used to perform the immunohistochemistry analysis for P16, with all markers displaying a similar nuclear immunoreactivity target | |
| • | BDA Pharminger performed the immunohistochemistry analysis for P16 antibody, with lot number P-710134 |
Control for IHC: Tissues known to express the antigen provided the positive control for every marker. While different antibodies that are being evaluated were left out of the reagent negative controls, tissues that are known not to express certain antibody markers were used in the negative control.
Scoring system for CIN:
| A = IHC (%) | B intensity of IHC reaction | Final score |
| 0 = 0% | 0 = No reaction | A+B = range from 0 to 6 |
| 1 = <30% | 1 = Weak | |
| 2 = 30-60% | 2 = Mild | |
| 3 = >60% | 3 = Strong |
where, A is the perentage or the proportion of tumor cells stained and B is the intensity of the tumor cells stained.
Final interpretation of the IHC scoring:
| 0/6 | = | Negative reaction | |
| 1/6, 2/6 and 3/6 | = | Low expression | |
| 4/6, 5/6 and 6/6 | = | High expression |
Clinical information obtained:
| • | Type of cancer diagnosis | |
| • | Site of the cancer | |
| • | Sex of patients |
RESULTS
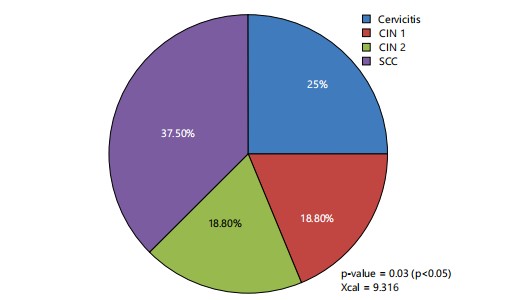
This study involved eighty (80) female subjects including cervicitis 25%, CIN 1, 18.8%, CIN 2, 18.8% and 37.50% for SCC as shown in Fig. 1.
Table 1 show the rate of expression of P16, among the cervicitis, 11(55.0%) showed positive expression against 9(45.0%) that had no expression with statistically insignificant (p>0.05) difference between them. Among the CIN 1 cases examined, 13(86.7%) had positive expression for P16 against 2(13.3%) that showed no expression with a significant (p<0.05) higher rate of positive expression, 9(60.0%) of the CIN 2 had positive expression against 6(40.0%) that showed no expression, there was a significant high 22(73.3%) expression of P16 among the SCC cases against 8(26.7%) that showed no expression.
|
|
|
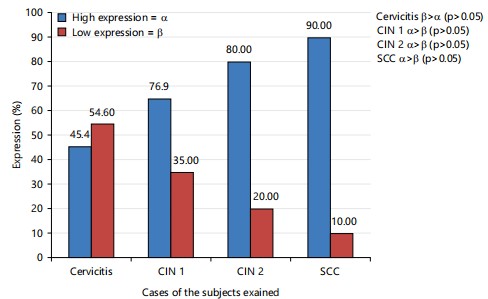
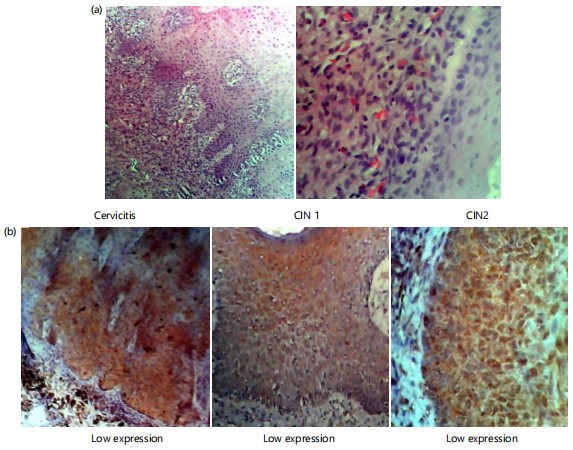
From the result (Fig. 2), there was a gradual increase in high expression from cervicitis to CIN 1, CIN 2 and SCC cases. There was a significant (p<0.05) high degree of expression of P16 in CIN 1, CIN 2 and SCC cases, while a statistically insignificant (p>0.05) difference in the expression of P16 was found in cervicitis cases. Fig. 3 expresses the photomicrograph of cervicitis slides stained with Heamatoxylin and Eosin.
| Table 1: | Rate of expression of P16 among the difference cases in the study | |||
| Diagnosis | Number examined | Positive expression n (%) | Negative expression n (%) | p-value |
| Cerviticitis | 20 | 11 (55.0) | 9 (45.0) | 0.317 |
| CIN 1 | 15 | 13 (86.7) | 2 (13.3) | <0.0001 |
| CIN 2 | 15 | 9 (60.0) | 6 (40.0) | 0.045 |
| SCC | 30 | 22 (73.3) | 8 (26.7) | <0.0001 |
| Total | 80 | 55 (68.8) | 25 (31.2) | 0.0002 |
| CIN: Intraepithelial neoplasm, SCC: Squamous cell carcinoma, %: Percentage of expression and p-value: Significant level | ||||
DISCUSSION
The current study stated the relationship between cervical neoplasia and p16 expression. The majority of dysplastic/neoplastic cells showed significant immunohistological expression of p16 compared to the normal cervical epithelium and cervicitis. Thus, establishing previous research that smaller series14, p16 expression seems to be a reliable, sensitive and specific biomarker of cervical neoplasia. While other possible explanations cannot be ruled out, inactivation of RB by high-risk HPVs is most likely the primary cause of elevated expression of p16 in the context of CIN. This hypothesis is supported in part by the finding that cervical specimens with higher grades of CIN or invasive carcinoma-lesions that are strongly linked to high-risk HPV infection-showed progressively higher p16 expression scores.
One can anticipate that the majority of CIN 1 instances and a sizable fraction of CIN 2 and CIN 3 cases will spontaneously regress17. Compared to the results of previous study by Fredrick et al.18, in numerous other instances, the dysplasia grade will exhibit steady persistence.
Proliferative potentials were found to be positively correlated with the pathogenesis of cervical cancer in current investigation in Fig. 2, 90.0%, CIN 2 80.0%, CIN 1 76.9% and 45.4% of cases of cervicitis according to Chinyere et al.19, very small percentages of women with CIN 1 and CIN 2 lesions and a somewhat bigger fraction of women with CIN 3 lesions will go on to get invasive cancer if treatment is not received. This was also confirmed by the expression of the photomicrograph of cervicitis in Fig. 3. This highlights the need for predictive biomarkers that can determine which women with cervical dysplasia have a higher chance of acquiring cancer or higher grades of CIN. The situation is made more difficult by the fact that many women who are diagnosed with cervical carcinoma have not previously been diagnosed with a pre-invasive cervical lesion19.
Merely 9% of females diagnosed with cervical carcinoma in our database had antecedently been reported with pre-invasive cervical abnormalities. Although other pathways cannot be ruled out, increased expression of p16 in the setting of CIN probably occurs mainly as a result of the inactivation of RB by high-risk HPVs. Circumstantial support for this premise comes from the observation that increasingly high p16 expression scores were seen in cervical specimens showing higher grades of CIN or invasive carcinoma, lesions known to be closely associated with high-risk HPV infection. Most cases of CIN 1 and a large proportion of cases of CIN 2 and CIN 3 can be expected to regress spontaneously19,20. Thus, p16 expression appears to be a robust, specific and sensitive biomarker of cervical neoplasia, confirming the results of previous smaller series. The study recommended that awareness programs regarding cervical cancer and its risk factors should be organized at different educational levels and in rural and urban areas. Screening of all married/sexually active women for HPV infection should be done. The PAP smear facilities should be available at all the health centers of the state. All the women of the state should be screened for HPV types by using advanced immunohistochemical methods. Clinicians should also recommend women for regular PAP smear screening. Vaccination of females against HPV types should be done.
CONCLUSION
Because of the relationship between p16 and HPV E7 inactivated RB protein and its eventual progression to cervical cancer, we concluded that immunohistochemical detection of p16 expression is not a diagnostic marker for cervicitis but rather a specific diagnostic marker of cervical dysplasia (CIN 1 and CIN 2) and cervical cancer. It may also serve as a surrogate marker for HPV infection. While P16 expression 90% achieved is also a diagnostic tool for SCC and can also be used in conjunction with E-cadherin for diagnosis of cervical lesion/cervical cancer. However, the study has shown that these four biomarkers are good tools for early cancer detection (cervical lesion/cervical cancer).
SIGNIFICANCE STATEMENT
A family of viruses known as the Human Papillomavirus (HPV) is incredibly widespread throughout the world. Of the more than 100 varieties of HPV, at least 14 are high-risk kinds or those that can cause cancer. The majority of people contract HPV soon after they start having sexual relations because the virus is primarily spread through sexual contact. Certain kinds of HPV infections contracted through sexual activity are the cause of cervical cancer. This study involves the use of immunological markers in identifying the various stages of cancer progression, as well as in diagnosing cancer, predicting prognosis and potentially serving as therapeutic targets for squamous cell carcinoma and cervicitis. The p16 immunoreactivity predicts high-risk HPV.
REFERENCES
- Firnhaber, C., N. Mayisela, L. Mao, S. Williams and A. Swarts et al., 2013. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PLoS ONE, 8.
- Holland, J.F., 2010. Holland-Frei Cancer Medicine. 8th Edn., PMPH-USA, Shelton, Connecticut, ISBN: 9781607950141, Pages: 2021.
- Arbyn, M., X. Castellsagué, S. de Sanjosé, L. Bruni, M. Saraiya, F. Bray and J. Ferlay, 2011. Worldwide burden of cervical cancer in 2008. Ann. Oncol., 22: 2675-2686.
- Castellsagué, X., 2008. Natural history and epidemiology of HPV infection and cervical cancer. Gynecologic Oncol., 110: S4-S7.
- Moghissi, K.S., 1972. The function of the cervix in fertility. Fertil. Sterility, 23: 295-306.
- Dubey, V., S. Mythirayee, R.K. Tiwari, U. Gaharwar, M. Pal and S. Reddy, 2016. Cervical mucus helps in the fertilization. World J. Pharm. Pharm. Sci., 5: 242-250.
- Sun, J. and A.C. Spradling, 2013. Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. eLife, 2.
- Mukama, T., R. Ndejjo, A. Musabyimana, A.A. Halage and D. Musoke, 2017. Women’s knowledge and attitudes towards cervical cancer prevention: A cross sectional study in Eastern Uganda. BMC Women's Health, 17.
- Kumar, V., A.K. Abbas, N. Fausto and R. Mitchell, 2007. Robbins Basic Pathology. 8th Edn., Elsevier Health Sciences, Amsterdam, Netherlands, ISBN: 9781437700664, Pages: 960.
- Crosbie, E.J., M.H. Einstein, S. Franceschi and H.C. Kitchener, 2013. Human papillomavirus and cervical cancer. Lancet, 382: 889-899.
- Gray, R.H., J.L. Simpson, A.C. Bitto, J.T. Queenan and C. Li et al., 1998. Sex ratio associated with timing of insemination and length of the follicular phase in planned and unplanned pregnancies during use of natural family planning. Hum. Reprod., 13: 1397-1400.
- Osol, G. and M. Mandala, 2009. Maternal uterine vascular remodeling during pregnancy. Physiology, 24: 58-71.
- Delnooz, C.C.S., J.W. Pasman, C.F. Beckmann and B.P.C. van de Warrenburg, 2015. Altered striatal and pallidal connectivity in cervical dystonia. Brain Struct. Funct., 220: 513-523.
- Dabbs, D.J., 2010. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications, 3rd Edn., Saunders, London, United Kingdom, ISBN: 978-1-4160-5766-6, Pages: 941.
- Kritpracha, K., J. Hanprasertpong, V. Chandeying, C. Dechsukhum and A. Geater, 2005. Survival analysis in advanced epithelial ovarian carcinoma in relation to proliferative index of MIB-1 immunostaining. J. Obstet. Gynaecol., 31: 268-276.
- Negri, G., F. Vittadello, F. Romano, A. Kasal and F. Rivasi et al., 2004. P16INK4a expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch., 445: 616-620.
- Östör, A.G., 1993. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol., 12.
- Fredrick, C.C., O.N.C. Ndidi, K.S. Nwokorie, D. Amaechi and J.U. Madukwe et al., 2023. Analysis and determination of the proliferative biomarker potential of Ki-67 in cervical lession/cervical cancer using immunohistochemical method. J. Coastal Life Med., 11: 1371-1381.
- Chinyere, F.C., D. Amaechi and M.L. Dansura, 2023. The Immunohistochemical expressions and diagnostic values of E-cadherin and β-Catenin in suspected cervical lesion or cervical cancer. J. Appl. Health Sci. Med., 3: 1-18.
- Klaes, R., T. Friedrich, D. Spitkovsky, R. Ridder and W. Rudy et al., 2001. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int. J. Cancer, 92: 276-284.
How to Cite this paper?
APA-7 Style
Chinyere,
F.C., Amaechi,
D., Ekpe,
I.P., Dansura,
M.L. (2024). Analysis of HPV in Cervical Lesion/Cervical Cancer Using P16 by Immunohistochemical Method. Asian Science Bulletin, 2(4), 375-384. https://doi.org/10.3923/asb.2024.375.384
ACS Style
Chinyere,
F.C.; Amaechi,
D.; Ekpe,
I.P.; Dansura,
M.L. Analysis of HPV in Cervical Lesion/Cervical Cancer Using P16 by Immunohistochemical Method. Asian Sci. Bul 2024, 2, 375-384. https://doi.org/10.3923/asb.2024.375.384
AMA Style
Chinyere
FC, Amaechi
D, Ekpe
IP, Dansura
ML. Analysis of HPV in Cervical Lesion/Cervical Cancer Using P16 by Immunohistochemical Method. Asian Science Bulletin. 2024; 2(4): 375-384. https://doi.org/10.3923/asb.2024.375.384
Chicago/Turabian Style
Chinyere, Fredrick, Christy, Dennis Amaechi, Ini Patrick Ekpe, and Mangpin Leviticus Dansura.
2024. "Analysis of HPV in Cervical Lesion/Cervical Cancer Using P16 by Immunohistochemical Method" Asian Science Bulletin 2, no. 4: 375-384. https://doi.org/10.3923/asb.2024.375.384

This work is licensed under a Creative Commons Attribution 4.0 International License.




