Exploring the Interdisciplinary Frontier: Advancements in Tissue Engineering, Biofluids and Bioinstrumentation
| Received 11 Mar, 2024 |
Accepted 14 May, 2024 |
Published 31 Dec, 2024 |
The fusion of mechanical engineering and medicine has revolutionized healthcare, particularly in tissue engineering, biofluid dynamics and bioinstrumentation. This research explores the collaboration between engineers and medical professionals, showing promise for overcoming challenges in tissue engineering. However, replicating complex tissue characteristics remains a significant hurdle in creating functional biological tissues for regenerative medicine and drug testing. Mechanical engineering plays a crucial role by providing the necessary tools to design functional biomaterials and measure essential physical parameters vital for tissue engineering. This paper focuses on the significance of biofluids and bioinstrumentation, exploring their pivotal functions. Biofluids, which include blood, lymph and interstitial fluid, are essential for nutrient transport, mechanical stress distribution and cell signaling. On the other hand, bioinstrumentation involves the development of devices for measuring and manipulating biological parameters, which are crucial for the characterization and monitoring of living tissues. The interdisciplinary collaboration between mechanical engineering and medicine has led to remarkable achievements, particularly in the areas of tissue regeneration and biofluid dynamics. Landmark studies conducted according to literature have shown the feasibility of tissue engineering, paving the way for the creation of synthetic organs. Simultaneously, advancements in biofluid dynamics, as demonstrated by other researchers, have provided valuable insights into the complexities of blood flow, guiding interventions in cardiovascular health. The integration of bioinstrumentation, as highlighted by Webster, has improved diagnostic capabilities, underscoring the transformative potential of interdisciplinary cooperation. This paper provides an overview of the current status and future prospects of biofluids and bioinstrumentation in tissue engineering. It includes a comprehensive literature review, discussing the various types and characteristics of biofluids and showcases recent advancements and emerging trends in the field. Special emphasis is placed on the synergistic relationship between mechanical engineering and medicine in driving innovation in healthcare.
INTRODUCTION
The confluence of mechanical engineering and medicine is reshaping the traditional boundaries of healthcare innovation. Engineers, equipped with advanced skills and technologies, are at the forefront of creating biomaterials that exhibit remarkable functionalities, ushering in a new era of medical treatments. This collaborative synergy between engineers and physicians holds great promise in addressing the complex challenges posed by tissue engineering, biofluid dynamics and bioinstrumentation. This paper explores the evolving landscape of this interdisciplinary frontier, shedding light on the transformative potential it holds for patient care.
Tissue engineering is a rapidly growing field that aims to create functional biological tissues and organs for various applications, such as regenerative medicine, drug testing and disease modeling as in Fig. 11. Tissue engineering involves the integration of cells, biomaterials and biophysical stimuli to mimic the structure and function of native tissues2. However, tissue engineering faces many challenges, such as reproducing the complexity and diversity of living tissues, ensuring the viability and functionality of engineered tissues and translating the results from the laboratory to the clinic3. To overcome these challenges, tissue engineering requires the collaboration of multiple disciplines, such as biology, chemistry, physics, engineering and medicine4,5.
In particular, mechanical engineering plays a crucial role in tissue engineering, as it provides the tools and methods to design and create functional biomaterials and to measure and manipulate the physical parameters of biological systems6. In this paper, the focus is on two key areas of mechanical engineering that are essential for tissue engineering: Biofluids and bioinstrumentation. Biofluids are fluids that flow within or around living tissues, such as blood, lymph and interstitial fluid. Biofluids are responsible for transporting nutrients, oxygen, waste and signaling molecules, as well as for exerting mechanical forces and stresses on the cells and the extracellular matrix7.
Bioinstrumentation is the development and use of devices and methods for measuring and manipulating biological parameters, such as pressure, flow, temperature and electrical signals. Bioinstrumentation enables the characterization and monitoring of the properties and behaviors of living tissues, as well as the delivery and stimulation of engineered tissues8.
Both biofluids and bioinstrumentation are essential for tissue engineering, as they enable the creation and evaluation of functional and realistic tissue constructs that can mimic the native tissue microenvironment and interact with the host organism9. The main objective of this paper is to review the current state of the art and the future directions of biofluids and bioinstrumentation for tissue engineering.
The overlap between mechanical engineering and medicine has manifested in remarkable achievements, with a surge in research focused on tissue engineering, biofluids and bioinstrumentation. Pioneering studies by Sharabi10 demonstrated the feasibility of tissue engineering11, paving the way for synthetic organs and tissues. Meanwhile, advancements in biofluid dynamics, as explored by Rutkowski and Swartz12, have unraveled intricacies in blood flow, guiding the design of cardiovascular interventions. The integration of bioinstrumentation, elucidated by Young and Christman13, has enhanced diagnostics and monitoring capabilities. Collectively, these contributions underscore the transformative potential of interdisciplinary collaboration.
A brief overview of the basic concepts and principles of biofluids and bioinstrumentation is provided and then the main challenges and opportunities of these two areas are discussed for tissue engineering. Next, highlighted some of the recent advances and emerging trends in biofluids and bioinstrumentation, such as microfluidics, organ-on-a-chip, wearable and implantable devices and biohybrid systems. Finally, the outlining of some of the open questions and future directions for biofluids and bioinstrumentation in tissue engineering are concluded.
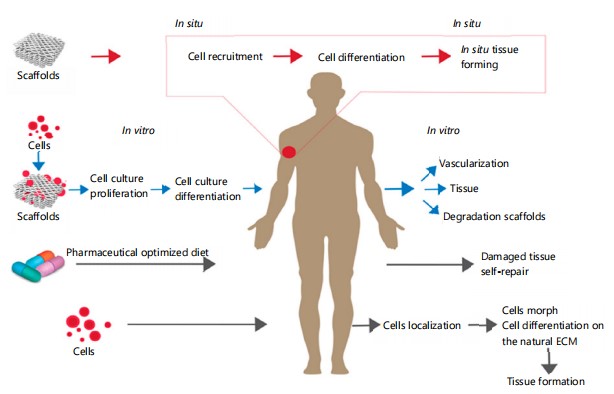
|
Literature review: This section provides a comprehensive review of the current literature on biofluids and bioinstrumentation for tissue engineering. The initial focus is on describing the primary types and characteristics of biofluids, followed by an in-depth discussion of the main methods and techniques used for biofluid analysis and manipulation. Subsequently, the main types and functions of bioinstrumentation are introduced, followed by a thorough examination of the methods and techniques employed in bioinstrumentation design and fabrication. Furthermore, this review includes examples and applications of biofluids and bioinstrumentation in tissue engineering, along with an analysis and discussion of the key challenges and limitations associated with the existing approaches.
Biofluids: Biofluids, encompassing blood, lymph and interstitial fluid, play a crucial role in transporting nutrients, oxygen, waste and signaling molecules within or around living tissues. This dynamic fluid system exerts mechanical forces and stresses on cells and the extracellular matrix, contributing to the complexity and heterogeneity of biofluid composition. Comprising water, electrolytes, proteins, lipids, carbohydrates, hormones and cells, biofluids respond dynamically to physiological and pathological conditions within an organism. Recent research underscores the importance of understanding and controlling the properties and interactions of biofluids in tissue engineering applications.
Notably, studies have delved into the intricate composition of biofluids, elucidating the role of specific biomolecules in cellular responses. Additionally, the work of Yazdanpanah et al.14 highlights the dynamic nature of biofluids, emphasizing their adaptability in various physiological contexts. Furthermore, recent findings underscore the relevance of mimicking native biofluid environments in tissue engineering for optimal growth and functionality of engineered tissues. These works collectively emphasize the significance of biofluids as essential components in tissue engineering, necessitating a nuanced understanding of their properties and behaviors for successful biomimetic applications.
Types and characteristics of biofluids: The main types of biofluids that are relevant for tissue engineering are blood, lymph and interstitial fluid. Blood is the fluid that circulates in the cardiovascular system, carrying oxygen and nutrients to the tissues and removing carbon dioxide and waste from the tissues. Blood is composed of plasma, which is a water-based solution of electrolytes, proteins, hormones and other molecules and blood cells, which are red blood cells, white blood cells and platelets. Blood has a density of about 1060 kg/m3, a viscosity of about 3–4 mPas and a pH of about 7.4. Blood flow is driven by the pressure gradient generated by the heart and regulated by vascular resistance, which depends on the geometry, elasticity and tone of the blood vessels. Blood flow is also influenced by the hematocrit, which is the ratio of blood cells to plasma and the Fahraeus-Lindqvist effect, which is the reduction of apparent blood viscosity in small vessels due to the migration of blood cells to the center of the vessel.
Blood flow can be laminar, turbulent, or transitional, depending on the Reynolds number, which is the ratio of inertial forces to viscous forces. Blood flow can also be pulsatile, steady, or oscillatory, depending on the frequency and amplitude of the pressure and flow variations. Blood flow is important for tissue engineering, as it affects the mass transport, shear stress and oxygen tension of the engineered tissues15.
Lymph is the fluid that circulates in the lymphatic system, collecting and returning the excess fluid, proteins and cells from the interstitial space to the blood. Lymph is composed of water, electrolytes, proteins, lipids, hormones and cells, mainly lymphocytes. Lymph has a density of about 1025 kg/m3, a viscosity of about 2 mPa s and a pH of about 7.6. Lymph flow is driven by the pressure gradient generated by the contraction of lymphatic vessels and the movement of surrounding tissues and regulated by the valves, which prevent the backflow of lymph. Lymph flow is also influenced by the lymphatic capillary permeability, which depends on the interstitial fluid pressure and the presence of anchoring filaments, which connect the lymphatic endothelium to the extracellular matrix. Lymph flow is usually slow, intermittent and unidirectional, with an average velocity of about 0.1-0.3 mm/s and a flow rate of about 2–4 L/day. Lymph flow is important for tissue engineering, as it affects the fluid balance, immune response and waste removal of the engineered materials.
Interstitial fluid is the fluid that fills the spaces between the cells and the extracellular matrix, providing the medium for diffusion and exchange of substances (Fig. 2). Interstitial fluid is composed of water, electrolytes, proteins, lipids, hormones and other molecules and has a similar composition to plasma, except for the lower protein concentration. Interstitial fluid has a density of about 1000 kg/m3, a viscosity of about 1.0 mPa s and a pH of about 7.4. Interstitial fluid flow is driven by the pressure gradient between the blood and the lymphatic capillaries16 and regulated by the starling forces, which depend on the hydrostatic and osmotic pressures of the fluid and the permeability of the capillary wall. Interstitial fluid flow is also influenced by the interstitial matrix structure, which can be porous, fibrous, or gel-like, depending on the tissue type and state. Interstitial fluid flow is usually slow, continuous and multidirectional, with an average velocity of about 0.01-0.1 mm/s and a flow rate of about 2–4 L/day. Interstitial fluid flow is important for tissue engineering, as it affects the nutrient and waste exchange, cell signaling and tissue remodeling of the engineered materials17.
Some of the main methods and techniques for biofluid analysis and manipulation are rheology, microscopy, spectroscopy, flow cytometry, microfluidics and bioreactors. Rheology is the study of the deformation and flow of matter and it can be used to measure the viscosity, elasticity and viscoelasticity of biofluids, as well as the effects of shear stress and strain on the biofluid components18-20.
Microscopy is the use of optical or electron instruments to magnify and visualize the structure and morphology of biofluids and their components, such as cells, proteins and lipids. Spectroscopy is the use of electromagnetic radiation to measure the absorption, emission or scattering of light by biofluids and their components and it can be used to determine the concentration, composition and interaction of biofluid molecules21,22.
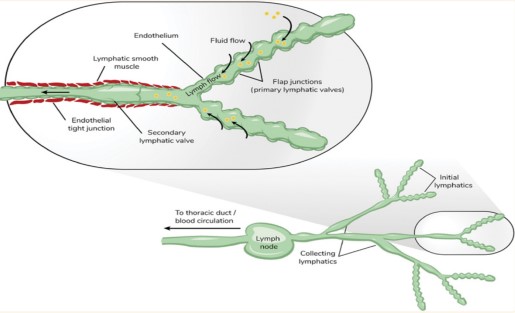
|
Flow cytometry is the use of laser beams and detectors to measure the physical and chemical properties of biofluid cells, such as size, shape, granularity and fluorescence and it can be used to identify, count and sort different cell types and subpopulations23. Microfluidics is the manipulation of fluids at the microscale, using devices with channels, valves, pumps and mixers and it can be used to create controlled and precise biofluid environments, such as blood vessels, lymphatic vessels and interstitial spaces24. Bioreactors are devices that provide a suitable environment for the growth and maintenance of biological systems, such as cells, tissues and organs and they can be used to expose engineered tissues to biofluid flow and other biophysical stimuli, such as pressure, temperature and electrical signals25.
Methods and techniques for biofluids and bioinstrumentation in tissue engineering
Design and fabrication
Materials: Different types of materials, such as biocompatible polymers like polydimethylsiloxane (PDMS), hydrogels and microfabricated materials, are commonly used in the design and fabrication of biofluidic devices. The selection of materials is based on their biocompatibility, mechanical strength and optical transparency properties.
Fabrication methods: The fabrication of intricate structures for biofluidic devices is made possible through the use of microfabrication techniques such as soft lithography and 3D printing. Precision machining, microfabrication, or additive manufacturing methods are often employed in the production of bioinstrumentation devices.
Integration strategies: The integration of sensors, actuators and other components into biofluidic systems is of utmost importance. Microfluidic channels and chambers are specifically designed to mimic physiological conditions, allowing for controlled experiments to be conducted. For instance:
| • | Sensor integration: Sensors, such as pressure sensors, temperature sensors and pH sensors, can be integrated into biofluidic devices to monitor and measure various parameters. These sensors are often miniaturized and integrated directly into the microfluidic channels or chambers to enable real-time monitoring | |
| • | Actuator integration: Actuators, such as microvalves and micropumps, are essential for controlling the flow of fluids within biofluidic devices. These actuators can be integrated into the device to precisely control the movement and direction of fluids, allowing for precise manipulation and analysis | |
| • | Optical integration: Optical components, such as lenses, filters and light sources, are often integrated into biofluidic devices to enable optical detection and analysis. These components can be designed and fabricated to allow for specific wavelengths of light to pass through the device, enabling fluorescence detection, absorbance measurements and imaging | |
| • | Electrical integration: Electrical components, such as electrodes and microfluidic interconnects, are integrated into biofluidic devices to enable electrical sensing and stimulation. These components can be designed and fabricated to provide electrical connections for external devices, such as power sources or data acquisition systems | |
| • | Software integration: Biofluidic devices often require software integration to control and analyze data. This can involve the development of user-friendly interfaces for device control, data visualization and data analysis. Software integration allows for automation, data storage and data processing, enhancing the functionality and usability of biofluidic devices | |
| • | Packaging and encapsulation: Once the biofluidic device is designed and fabricated, it needs to be packaged and encapsulated to protect it from external contaminants and ensure its long-term stability. Packaging can involve the use of biocompatible materials, such as glass or polymers and may include sealing techniques, such as bonding or encapsulation with biocompatible adhesives |
Overall, the design and fabrication of biofluidic devices involve the careful selection of materials, the use of microfabrication techniques and the integration of various components to create functional and reliable devices for biomedical research, diagnostics and therapeutic applications.
Characterization and testing
Measurement and imaging techniques: Techniques such as micro-particle image velocimetry (μPIV), fluorescence microscopy and atomic force microscopy are employed to visualize and quantify fluid flow, mechanical forces and cellular responses.
Standards and benchmarks: Characterization involves comparing device performance against established standards. For biofluidic devices, benchmarks may include flow rates, shear stresses and mass transport capabilities. Bioinstrumentation devices are often assessed for accuracy, sensitivity and response time.
The following testing methods should be well noted:
| • | Cell viability and functionality testing: Biofluids and bioinstrumentation in tissue engineering must also be tested for their impact on cell viability and functionality. This can involve assays such as cell viability staining, metabolic activity assays and gene expression analysis | |
| • | Physical and mechanical testing: Biofluids and bioinstrumentation devices are subjected to physical and mechanical tests to assess their durability, stability and performance under different conditions. This may involve testing for pressure resistance, temperature sensitivity and material integrity. The mechanical properties of biofluidic devices and bioinstrumentation are crucial for their functionality in tissue engineering applications. Techniques such as tensile testing, compression testing and rheological analysis can be used to assess the strength, stiffness and flexibility of these devices | |
| • | Biological testing: In tissue engineering, biofluids and bioinstrumentation devices must also be tested for their compatibility with biological systems. This includes assessing their biocompatibility, cytotoxicity and ability to support cell growth and function | |
| • | Computational modeling: Computational modeling techniques are used to simulate and predict the behavior of biofluids and bioinstrumentation devices. This allows researchers to optimize device design, predict performance outcomes and understand complex fluid dynamics and cellular interactions | |
| • | In vitro and in vivo testing: Biofluids and bioinstrumentation devices are often tested in both in vitro (laboratory) and in vivo (animal or human) settings to evaluate their performance and safety. In vitro testing allows for controlled experiments, while in vivo testing provides more realistic physiological conditions | |
| • | Long-term stability and reliability testing: To ensure the long-term success of biofluids and bioinstrumentation devices in tissue engineering applications, researchers conduct stability and reliability testing over extended periods of time. This involves monitoring device performance, functionality and biocompatibility over weeks, months, or even years |
Application in tissue engineering
Cell types: Various cell types, including stem cells, primary cells and tissue-specific cells, are incorporated into biofluidic systems to simulate physiological conditions. This aids in understanding cellular responses to fluidic environments.
Biomaterials: Engineered biomaterials, such as scaffolds and hydrogels, are integrated into biofluidic systems to create tissue-like environments. These materials provide structural support and influence cellular behavior.
Biophysical stimuli: Controlled application of biophysical stimuli, including fluid shear stress and mechanical forces, guides tissue development. Integration of bioinstrumentation allows real-time monitoring and adjustment of these stimuli.
Biofluids, such as blood and cerebrospinal fluid, are not only used as a medium to culture cells in biofluidic systems but also serve as a source of nutrients and oxygen for the cells. These fluids closely mimic the physiological conditions found in the human body, providing a more realistic environment for studying cell behavior and tissue development.
Bioinstrumentation, including microfluidic devices and bioreactors, is used to precisely control the flow of biofluids and apply specific mechanical forces to the cells. These instruments allow researchers to create dynamic and physiologically relevant conditions, such as pulsatile flow or cyclic strain, which are crucial for tissue engineering applications. By mimicking the mechanical cues that cells experience in vivo, bioinstrumentation helps promote cell differentiation, tissue organization and overall tissue functionality.
The integration of biofluids and bioinstrumentation also enables the study of cell-cell and cell-matrix interactions within tissue-engineered constructs. By culturing different cell types in close proximity and exposing them to specific fluidic conditions, researchers can investigate how cells communicate and collaborate to form complex tissues. This knowledge is vital for designing biomaterials and scaffolds that can support the growth and organization of multiple cell types, leading to the development of functional tissues with enhanced physiological properties.
Biofluidic systems also offer the advantage of scalability, allowing for the production of large quantities of tissue-engineered constructs. By optimizing the flow rates, nutrient supply and waste removal in these systems, researchers can achieve high cell densities and promote tissue growth on a larger scale. This scalability is crucial for the eventual translation of tissue engineering technologies into clinical applications, where the production of functional tissues in sufficient quantities is essential.
The combination of biofluids and bioinstrumentation in tissue engineering also opens up new possibilities for drug screening and personalized medicine. By culturing patient-specific cells in biofluidic systems, researchers can evaluate the response of these cells to different drugs or therapeutic interventions. This approach allows for the development of personalized treatment strategies, where the efficacy and safety of drugs can be tested on a patient's cells before administration.
In brief, the integration of biofluids and bioinstrumentation in tissue engineering provides a powerful platform for studying cell behavior, guiding tissue development and advancing regenerative medicine. By mimicking the natural environment of tissues and precisely controlling the fluidic conditions, researchers can gain valuable insights into cell responses, optimize biomaterials and ultimately develop functional tissues for various applications in healthcare.
RESULTS AND OUTCOMES
Experimental data and simulation insights: The investigation into Methods and Techniques for Biofluids and Bioinstrumentation in Tissue Engineering has yielded substantial results. Experimental data illustrates the efficacy of biofluidic and bioinstrumentation devices in replicating physiological conditions. Simultaneously, simulations utilizing computational fluid dynamics (CFD) and finite element analysis (FEA) provide additional insights, contributing to the optimization of these devices.
Applications, successes and acknowledging limitations: The successful deployment of these devices in tissue engineering demonstrates their potential in constructing biomimetic environments for cell culture and tissue development. However, it is imperative to acknowledge inherent limitations, particularly in addressing challenges related to scaling and reproducing intricate tissue architectures.
Multidisciplinary approach for advancements: In summary, the conception, creation, assessment and application of biofluidic and bioinstrumentation devices in tissue engineering demand a multidisciplinary strategy. The integration of diverse materials, fabrication techniques and testing methodologies remains pivotal in crafting functional systems that authentically emulate physiological conditions, thereby propelling advancements in the regenerative medicine arena.
Moreover, the exploration of various biofluids, such as blood and interstitial fluid, enhances our comprehension of their role in tissue engineering. Analyzing the composition and flow characteristics of these biofluids enables the design of bioinstrumentation devices capable of accurately mimicking dynamic conditions within the human body.
Furthermore, the study underscores the significance of computational modeling in refining the design and functionality of biofluidic and bioinstrumentation devices. Through computational fluid dynamics (CFD) and finite element analysis (FEA), researchers simulate and analyze fluid flow patterns, mechanical stresses and mass transport phenomena. This computational approach proves instrumental in optimizing device performance and functionality.
The application of biofluidic and bioinstrumentation devices in tissue engineering signifies a transformative potential. These devices offer crucial physical and chemical cues supporting cell growth, differentiation and tissue regeneration. By faithfully reproducing physiological conditions, these devices stand to revolutionize regenerative medicine.
However, recognizing the limitations of biofluidic and bioinstrumentation devices is paramount. Scaling and reproducing intricate structures, especially of complex tissues like organs, pose substantial challenges. Despite the promises of biomimicry, addressing these complexities requires continued research and development efforts.
CONCLUSION
The multifaceted nature of developing and applying biofluidic and bioinstrumentation devices in tissue engineering necessitates a multidisciplinary approach. The integration of materials, fabrication methods and testing techniques is fundamental in creating functional systems that faithfully replicate physiological conditions. This interdisciplinary synergy, intertwining engineering, biology and medicine, holds immense potential for advancing regenerative medicine. Ongoing advancements in research and development are paving the way for biofluidic and bioinstrumentation devices to transform tissue engineering and play a crucial role in the development of novel treatments for a wide range of diseases and injuries.
SIGNIFICANCE STATEMENT
The objective of this study was to investigate the intersection of mechanical engineering and medicine in advancing healthcare, particularly in tissue engineering. Present research aimed to highlight the transformative potential of interdisciplinary collaboration in overcoming tissue engineering challenges. Through reviewing current literature and presenting examples, this study underscored the crucial role of biofluids and bioinstrumentation in tissue engineering applications. Current study contributes to the academic understanding by emphasizing the interdisciplinary synergy and showcasing recent advancements, ultimately paving the way for further innovations in regenerative medicine and personalized healthcare.
REFERENCES
- Abdulghani, S. and G.R. Mitchell, 2019. Biomaterials for in situ tissue regeneration: A review. Biomolecules, 9.
- Langer, R. and J.P. Vacanti, 1993. Tissue engineering. Science, 260: 920-926.
- Griffith, L.G. and G. Naughton, 2002. Tissue engineering-current challenges and expanding opportunities. Science, 295: 1009-1014.
- Nerem, R.M., 1991. Cellular engineering. Ann. Biomed. Eng., 19: 529-545.
- Sharma, P., P. Kumar, R. Sharma, V.D. Bhatt and P.S. Dhot, 2019. Tissue engineering; current status & futuristic scope. J. Med. Life, 12: 225-229.
- Liu, V.A. and S.N. Bhatia, 2002. Three-dimensional photopatterning of hydrogels containing living cells. Biomed. Microdevices, 4: 257-266.
- Pries, A.R. and T.W. Secomb, 2005. Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart Circ. Physiol., 289: H2657-H2664.
- Clark, J.W., 2010. Medical Instrumentation: Application and Design. 4th Edn., John Wiley and Sons, Hoboken, New Jersey, ISBN: 9788126553792, Pages: 713.
- Madden, P.W., J.N.X. Lai, K.A. George, T. Giovenco, D.G. Harkin and T.V. Chirila, 2011. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials, 32: 4076-4084.
- Sharabi, M., 2021. Structural mechanisms in soft fibrous tissues: A review. Front. Mater., 8.
- Goswami, A.K., M.S. Khaja, T. Downing, N. Kokabi, W.E. Saad and B.S. Majdalany, 2020. Lymphatic anatomy and physiology. Semin. Interventional Radiol., 37: 227-236.
- Rutkowski, J.M. and M.A. Swartz, 2007. A driving force for change: Interstitial flow as a morphoregulator. Trends Cell Biol., 17: 44-50.
- Young, D.A. and K.L. Christman, 2012. Injectable biomaterials for adipose tissue engineering. Biomed. Mater., 7.
- Yazdanpanah, G., Y. Jiang, B. Rabiee, M. Omidi and M.I. Rosenblatt et al., 2021. Fabrication, rheological, and compositional characterization of thermoresponsive hydrogel from cornea. Tissue Eng. Part C: Methods, 27: 307-321.
- Paxton, N.C., J. Ren, M.J. Ainsworth, A.K. Solanki and J.R. Jones et al., 2019. Rheological characterization of biomaterials directs additive manufacturing of strontium-substituted bioactive glass/polycaprolactone microfibers. Macromol. Rapid Commun., 40.
- Angelopoulos, I., M.C. Allenby, M. Lim and M. Zamorano, 2020. Engineering inkjet bioprinting processes toward translational therapies. Biotechnol. Bioeng., 117: 272-284.
- Hochstetter, A., 2020. Lab-on-a-chip technologies for the single cell level: Separation, analysis, and diagnostics. Micromachines, 11.
- Valizadeh, A. and A.Y. Khosroushahi, 2015. Single-cell analysis based on lab on a chip fluidic system. Anal. Methods, 7: 8524-8533.
- Wernérus, H., P. Samuelson and S. Ståhl, 2003. Fluorescence-activated cell sorting of specific affibody-displaying staphylococci. Appl. Environ. Microbiol., 69: 5328-5335.
- Zhang, Y.S., J. Aleman, A. Arneri, S. Bersini and F. Piraino et al., 2015. From cardiac tissue engineering to heart-on-a-chip: Beating challenges. Biomed. Mater., 10.
- Borah, A. and D.S. Kumar, 2022. Overcoming the Barriers of Two-Dimensional Cell Culture Systems With Three-Dimensional Cell Culture Systems: Techniques, Drug Discovery, and Biomedical Applications. In: Biomedical Product and Materials Evaluation: Standards and Ethics, Mohanan, P.V. (Ed.), Woodhead Publishing, Cambridge, England, ISBN: 9780128239667, pp: 179-229.
- Wang, Y., R.K. Kankala, C. Ou, A. Chen and Z. Yang, 2022. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioactive Mater., 9: 198-220.
- Barrs, R.W., J. Jia, S.E. Silver, M. Yost and Y. Mei, 2020. Biomaterials for bioprinting microvasculature. Chem. Rev., 120: 10887-10949.
- Huang, G., F. Li, X. Zhao, Y. Ma and Y. Li et al., 2017. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev., 117: 12764-12850.
- Peng, G., H. Liu and Y. Fan, 2017. Biomaterial scaffolds for reproductive tissue engineering. Ann. Biomed. Eng., 45: 1592-1607.
How to Cite this paper?
APA-7 Style
Erheyovwe,
B.P. (2024). Exploring the Interdisciplinary Frontier: Advancements in Tissue Engineering, Biofluids and Bioinstrumentation. Asian Science Bulletin, 2(4), 409-418. https://doi.org/10.3923/asb.2024.409.418
ACS Style
Erheyovwe,
B.P. Exploring the Interdisciplinary Frontier: Advancements in Tissue Engineering, Biofluids and Bioinstrumentation. Asian Sci. Bul 2024, 2, 409-418. https://doi.org/10.3923/asb.2024.409.418
AMA Style
Erheyovwe
BP. Exploring the Interdisciplinary Frontier: Advancements in Tissue Engineering, Biofluids and Bioinstrumentation. Asian Science Bulletin. 2024; 2(4): 409-418. https://doi.org/10.3923/asb.2024.409.418
Chicago/Turabian Style
Erheyovwe, Bubu, Pius.
2024. "Exploring the Interdisciplinary Frontier: Advancements in Tissue Engineering, Biofluids and Bioinstrumentation" Asian Science Bulletin 2, no. 4: 409-418. https://doi.org/10.3923/asb.2024.409.418

This work is licensed under a Creative Commons Attribution 4.0 International License.




