Improving the Quality of Injera by Optimizing the Mixing Proportion of Fenugreek, Sorghum and Teff Flour
| Received 20 Jun, 2024 |
Accepted 28 Sep, 2024 |
Published 30 Sep, 2024 |
Background and Objective: Protein energy and micro-macronutrient malnutrition is a main global public health concern affecting humans worldwide, especially in Africa. To overcome this problem food products made from composite flours are required. Therefore, this study was conducted to find out the regression model and maximum mixing proportions of fenugreek, sorghum and teff flour that result in injera with higher quality attributes. Materials and Methods: A D-optimal mixture design with 3 factors and 2 levels was employed. A total of 14 baking trials were created using mixtures of fenugreek (0-5%), sorghum (0-50%) and teff (50-100%). The maximum and minimum values of independent variables were first evaluated by performing a preliminary study. Staling rate, mineral, protein, fiber, carbohydrate, antinutritional components and sensory qualities were all considered as response variables in this study. Results: That increasing the proportion of fenugreek flours in injera made from teff-sorghum-fenugreek mixing ratios improved nutritive values, improved sensory appeal, textural characteristics and reduced staling rate. Conclusion: The best injera blending ratio was found to be 64.1% teff, 32% sorghum and 3.80% fenugreek in terms of overall qualitative attributes. This could ultimately help to avail nutritionally improved, reduced staling rate and acceptable injera to the consumer.
INTRODUCTION
Protein-energy malnutrition and micro-macronutrient malnutrition represent the most serious public health issues in many developing countries, particularly in Africa1. It is a significant burden linked to poor health and physical function. Increasing the amount of dietary protein and micro-macronutrients in staple foods is critical. Composite readily available and highly nutritious foods provide an inexpensive way of preventing and alleviating this condition. Cereals are known to be high in carbohydrates and dietary fiber while having low protein content. To improve the protein-energy and micro-nutrient demands of emerging nations, food products made from a composite of cereals and legumes are required.
Injera is a traditional leavened bread from Ethiopia that is fermented, soft and round with eyes on the top surface due to carbon dioxide (CO2) production during fermentation and baking2-4. Teff (Eragrostis tef (Zucc.) Trotter) is a small cereal grain that is richer in essential nutrients than common cereal grains (such as wheat, barley, sorghum, maize and rice) due to its utilization as a whole grain5,6. Many Ethiopians make injera from teff far more frequently than from any other source and eat on a daily basis7.
Sour taste, smooth, moist, elastic, spongy feel, multiple “eyes” (bubbles) and a long shelf life are the indicators of quality injera. Teff is advised for celiac patients due to its lack of gluten and excellent potential as a functional meal. It is high in B vitamins and minerals and contains larger quantities of essential amino acids than wheat and barley, making it a rich source of those nutrients8. Injera can be produced from a range of cereals, such as teff, barley, sorghum, maize and wheat or a combination of various cereal flours9. Teff, is less accessible and affordable to low-income urban and pre-urban society and thus is primarily consumed in metropolitan areas with higher household incomes7. As a result, currently, most Ethiopians cannot afford to prepare injera made from teff alone and it is unrealistic to anticipate a drop in the price of teff even in the near future. Therefore, to lessen Ethiopia's existing food issues, it is vital to support the partial substitution of affordable and abundant cereals for teff, which has already begun. Like other grains, sorghum (Sorghum bicolor L. Moench) is a great source of protein and starch and is the main source of food for a sizable portion of the population in semi-arid tropical areas. It is a gluten-free grain, which is important in today’s world, where the prevalence of celiac disease, an immune response to gluten intolerance, is on the rise. The sorghum mix flour injera recipe, on the other hand, has poor injera-making qualities, such as staling and fragile texture during storage10.
The uncontrolled staling of sorghum injera results in limited softness, freshness, rollability, brittleness and dryness creating significant problems during storage. The study carried out by Yetneberk et al.2, reported that good injera could be prepared from teff-sorghum mix at a ratio of 50:50 and the authors concluded that injera quality continued to improve as the composite’s teff flour content surpassed 50%. However, there are limited research reports on the value addition of sorghum flour mix injera.
Given that fenugreek (Trigonella foenum-graecum L.) offers a variety of health advantages in addition to nutritional value, it is regarded as a primary functional food ingredient11. Fenugreek is a legume family annual herb that contains more protein than cereal grains making it a preferable crop to supplement cereal grains during injera making12.
It functions as a fortified component and a plasticizer, giving the essential dough homogeneity and crumb elasticity, lowering the rate of staling and increasing the nutritional content of baked products. It also has carminative, analgesic, antipyretic, anticancer and antioxidant properties, as well as anti-diabetic, anti-fertility, antibacterial, antiparasitic and antiseptic properties13. Fenugreek seeds consist of 45 to 60% carbohydrates, in which mucilaginous fibre (galactomannans), 20 to 30% proteins high in tryptophan and lysine, 5 to 10% fixed oils (lipids), pyridine alkaloids, mainly choline (0.5%), trigonelline (0.2-0.38%), gentianine and carpaine, the flavonoids apigenin orientin, luteolin, quercetin, vitexin and isovitexin, free amino acids, such as 4-hydroxyisoleucine (0.09%), arginine, lysine and histidine calcium and iron, saponins (0.6-1.7%), glycosides yielding steroidal sapogenins on hydrolysis (diosgenin, yamogenin, tigogenin, neotigogenin), cholesterol and sitosterol, vitamins B, A, C and nicotinic acid and 0.015% volatile oils (n-alkanes and sesquiterpenes)14.
Fenugreek also contains large amounts of a variety of derived metabolites, including tannins, terpenoids, alkaloids and flavonoids, which have been demonstrated in vitro to exhibit antimicrobial effects. Phenolic compounds aid plants in warding off bacteria, viruses, fungi and insects in addition to signaling molecules, tastes and colors that can attract or repel pests15. Fenugreek is added to teff-sorghum flour when making injera to enhance the organoleptic qualities, sensory appeal, baking quality and shelf life. Several studies have shown that injera-made cereal flours enriched with fenugreek improve the injera’s protein composition and sensory attributes12,16.
Due to the problems described above, the need for mixing sorghum with teff and fenugreek is increasing more than ever before. However, for the required quality attributes of injera, the blending of these cereal flours must be in the right proportion and efforts made toward optimization are needed. To improve the qualitative features of injera derived from these cereals, this research aimed to identify the regression equation of injera formulation and the ideal mixing ratio of teff, sorghum and fenugreek flours.
MATERIALS AND METHODS
Sample collection and preparation: Materials used for laboratory analysis consisted of 0.112 kg of fenugreek (variety Burka), 1.256 kg of sorghum (variety Melkam) and 4.232 kg of teff (variety Boni) seeds obtained from Ethiopian Institute of Agricultural Research/centers, Ethiopia, grown in the main season 2021/2022. The study was carried out from February, 2022 to July, 2022. A coffee grinder mill (XFYC810, China) was used to grind fenugreek and a Perten Laboratory Mill (PM 120, Finland) was used to grind sorghum and teff, which were then sieved through a 0.50 mm fine sieve. After that, the matching flours were sealed off in polyethylene bags and kept dry until more testing was done. The laboratory activities were conducted at the Food Science and Nutrition Research Laboratories of Kulumsa, Melkassa and Debrezeit Agricultural Research Centers, EIAR head quarter quality research laboratory, the Centre for Food Sciences and Nutrition Lab and the Department of Applied Chemistry at Addis Ababa University.
Experimental design and composite flour formulation: A D-optimal mixture design with three factors and two levels was employed. The maximum and minimum values of independent variables were first evaluated by performing a preliminary study. The three independent variables (factors) used were teff flour (X1:A) in the range of 50 to 100%, sorghum (X2:B) in the range of 0 to 50% and fenugreek (X3:C) in the range of 0 to 0.5% and the dependent variables (responses) were proximate composition, mineral, anti-nutritional factors, staling rate, alkaline water retention capacity and sensorial attributes.
To reduce residual errors, the expanded design was employed to reproduce vertices and binary mixes at the edges. That is, the greatest coded value of the other two mixture components added to the smallest coded value of the first component of the combination equals one. The greatest coded value of the other two combination components added to the largest coded value of the first component also equals one.
To create test formulations and analyze the results, the software Design-Expert version 13.0.5.0 was used. As shown in Table 1 a total of 14 baking trials were generated-six for model points, four for lack of fit estimation and four replicates in random order-and the response parameters were evaluated. To determine the link between the dependent variable (Y) and the independent variable (X), a second-order polynomial model of the following form was fitted:
Y = β0+β1X1+β2X2+β3X3+β11X12+β22X22+β33X32+β12X1X2+β13X1X3+β23X2X3+ε |
(1) |
where, β0, β1-β3, β11-β33 and β12-β23 are the regression coefficients of the constant, linear, quadratic and interaction terms, respectively. The X1-X3 are coded independent variables, X1X2-X2X3 are interaction terms, ε is an error term and Y is the dependent variable.
The formulation for the teff, sorghum and fenugreek-containing flour composite blends (Table 1). A limited mixture D-optimal design based on these formulations was created. Each dry ingredient was blended consistently to homogenize it before being placed in a clean, tight-fitting plastic container and maintained at room temperature (25±2°C) until it was used. Among these 14 trials, trials 1 and 10, 2 and 12, 3 and 5, 13 and 14 are similar.
| Table 1: | D-optimal coded design for injera preparation from teff, sorghum and fenugreek | |||
| Xi = Factors (independent variables) | |||
| Formulation/trial | X1 = A: Teff | X2 = B: Sorghum | X3 = C: Fenugreek |
| T1 | 0.95 | 0.00 | 0.05 |
| T2 | 1.00 | 0.00 | 0.00 |
| T3 | 0.75 | 0.25 | 0.00 |
| T4 | 0.87 | 0.12 | 0.01 |
| T5 | 0.75 | 0.25 | 0.00 |
| T6 | 0.84 | 0.12 | 0.04 |
| T7 | 0.62 | 0.37 | 0.01 |
| T8 | 0.73 | 0.24 | 0.03 |
| T9 | 0.62 | 0.34 | 0.04 |
| T10 | 0.95 | 0.00 | 0.05 |
| T11 | 0.5 | 0.45 | 0.05 |
| T12 | 1.00 | 0.00 | 0.00 |
| T13 | 0.50 | 0.50 | 0.00 |
| T14 | 0.50 | 0.50 | 0.00 |
Preparation of fermented dough and baking: The injera was made using the traditional teff dough preparation method6 with minor modifications17.
Determination of proximate composition
Moisture content: The moisture content of each sample was evaluated using the standard procedures17.
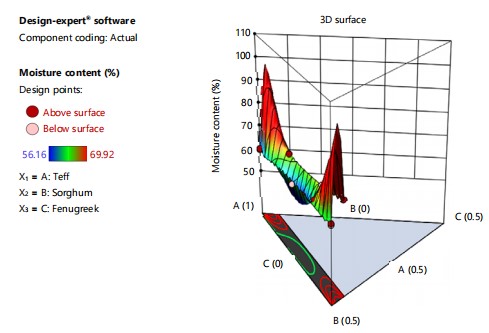
Total ash: The ash content of injera which measures the mineral composition of the injera was determined using Eq. 2 according to the Association of Official Analytical Chemistry using method 923.0318, by taking about 2.5 g sample (in duplicate) after carbonization and ignition at 550°C for 3 hrs in the muffle furnace (Nobertherm, Germany):
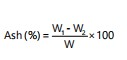 |
(2) |
where, W1 is the weight of the ash+crucible after ashing, W2 is the weight of the empty crucible and W is the weight of the sample.
Fat content: The AOAC (2000) official method 4.5.01 was used to calculate crude fat18. In the Soxhlet extractor (Model: DW-SXT-06, Chongqing, China), 2 g of injera sample (in duplicate) was extracted with 50 mL petroleum ether or diethyl ether for a minimum of 4 hrs. The solvent was then removed and the extracted fat was baked and chilled in a desiccator. The crude fat was calculated using Eq. 3:
| (3) |
where, W1 is the weight of the extraction flask, W2 is the weight of the extraction flask plus dried crude fat and Sw is the weight of the sample.
Crude fiber content:
The crude fiber content of the injera samples was evaluated using Eq. 417.
| (4) |
where, W1 is the weight of the crucible containing the sample after drying; W2 is the weight of the crucible with the sample after ashing and W3 is the fresh sample weight.
Determination of crude protein: The Kjeldahl method was used to quantify protein content in accordance with AOAC using the recognized method 920.8718. A 0.5 g sample of dried injera flour was obtained, mixed with 6 mL of an acid mixture (ortho-phosphoric acid and concentrated sulfuric acid) and then 3.5 mL of 30% hydrogen peroxide was gradually added. After that, 3 g of the catalyst solution -0.5 g of selenium metal and 100 g of potassium sulfate-was poured into several test tubes and left to stand for around 10 min. The digestion was then permitted to continue until a clear solution was attained. Around 25 mL of de-ionized water was added and the mixture was agitated to prevent sulfate precipitation. To begin the distillation process, 25 mL of boric acid and 25 mL of distilled water were poured into a 250 mL conical flask. The digested solution was transferred to the distiller’s sample container. A 35% sodium hydroxide solution (40 mL) was added to the digested and diluted solution. The distillation process was repeated for 9 min until a total volume of 200 to 250 mL was obtained. Finally, 0.1 N hydrochloric acid was used to titrate the distillate until a reddish color developed. After calculating the nitrogen% from the titration method, the crude protein was determined using Eq. 5:
Protein (%) = 6.25×Nitrogen (%) |
(5) |
Utilizable carbohydrate: The prepared injera’s carbohydrate content was calculated by the method of difference according to Tura et al.19:
Carbohydrate (%) = 100-[Moisture (%)+Protein (%)+Fat (%)+Ash (%)] |
Gross food energy: The difference approach was used to compute the gross energy using Eq. 6 as Atwater’s conversion factors20:
Energy (kcal/g) = (Carbohydrate (%)-crude fiber (%)×4+(crude fat (%)×9)+(crude protein (%)×4) |
(6) |
Determination of minerals (Fe, Zn and Ca): Using Eq. 7 and data from a flame atomic absorption spectrophotometer, the samples’ iron, zinc and calcium concentrations were computed (Model No. AAS-700, Perkin Elmer) and each formulated injera sample was analyzed in accordance with AOAC using the recognized method 985.3518. After obtaining ash using Eq. 3, a blank solution was made in a 50 mL volumetric flask using the same method as for the minerals reading. The sample reading was then conducted. Preparation of standard solutions: Six sets of working standard metal solutions (0.0, 0.5, 1, 1.5, 2.0 and 2.5 ppm for Ca and 0.0, 0.5, 1.0, 2.0, 3.0 and 4.0 ppm for Fe and 0.000, 0.125, 0.250, 0.500, 0.750 and 1.000 for Zn) of the metal stock solution was properly diluted to prepare the minerals (nitrate of the metal) with deionized water in 10 mL volumetric flask. The calibration curve (concentration vs. absorbance) for each element was derived using the absorbance process. Finally, the mineral element content was calculated as follows:
| (7) |
where, W is the weight of the sample on a dry matter basis, V is the volume of extract in liters, A is the concentration (mg/L) of sample solution, B is the concentration (mg/L) of blank solution and Df is the dilution factor (50 mL for Ca, Fe and Zn).
Determination of anti-nutritional factors
Condensed tannin content: The condensed tannin was evaluated using vanillin-HCL assay methods and a UV-VIS Spectrophotometer (Shimadzu, UV mini 1240, Japan), as described by Burns21 and revised by Maxson and Rooney22 grain chemistry. A gram of sample was measured in a screw cap test tube and
10 mL of 1% HCl in methanol was added to the tube holding the material to be tested. The sample-containing tube was shaken for 24 hrs at room temperature using a mechanical shaker (KS501 digital, ink laboratory CHNIK). The tube was then centrifuged for 5 min at 1000 rpm. In another test tube, 1 mL of supernatant was combined with 5 mL of vanillin-HCl. Finally, the specimen was left to sit for 20 min to allow the reaction to complete before measuring the absorbance of the colored intensity of the sample with a UV-visible spectrophotometer at 500 nm.
Preparation of standard solutions: A 40 mg D-catechin reference was mixed in 1000 mL of 1% HCl solution in methanol and standard solutions (0, 0.2, 0.4, 0.6, 0.8 and 1 mL) were taken in a test tube. The absorbance of standard solutions was determined at 500 nm with a UV-VIS Spectrophotometer (Shimadzu, UV mini 1240, Japan) following 20 min. The D-catechin calibration was utilized to quantify the concentration of condensed tannins. The tannin content was further evaluated as follows:
| (8) |
where, As is absorbance of sample solution, Ab is the blank absorbance, Int. is intercept from the absorbance equation curve, D is density of solution (0.791 g/mL) and W is weight of the sample in grams and 10 is the aliquot.
Phytate content: The phytate was determined using a modified colorimetric method23,17.
Determination of staling rate and alkaline water retention capacity: Equation (9) was used to calculate the samples’ alkaline water retention capacity (AWRC), which was modified by Licciadello et al.24. Dissolving 8.4 g of sodium bicarbonate in 1 L of water yielded a reagent containing 0.1 N sodium bicarbonate (NaHCO3) solution. Weighing 1 g of samples (in duplicate) and transferring them to 15 mL tubes (W1) allowed us to calculate the AWRC. Next, 5 mL of 0.1 N NaHCO3 was added, stirred and let the mixture sit at room temperature for 20 min. After centrifuging the slurry for 15 min at 3000 rpm and discarding the supernatant, the tubes were allowed to drip for ten minutes. The dried tubes were then weighed (W2):
| (9) |
where, W1 is weight of the tube containing the dry sample and W2 is weight of the tube containing the dripped sample. Then staling rate is calculated as follows:
| (10) |
where, AWRC0 denotes AWRC at zero time and AWRCn denotes AWRC on a certain storage day.
pH: A digital pH meter (pH-3CB, Changzhou, Jiangsu, China) was used to determine the pH of the specimens. The pH meter was adjusted using standard buffering solutions at pH 4 and 7 and each injera suspension (a well-homogenized mixture of 10 g ground injera and 100 mL distilled water) was determined25.
Sensory attributes: A preliminary sensory acceptability test of injera was performed to identify the most acceptable sorghum-teff-fenugreek substitution level in the injera-formulation process. The sensory analysis of injera samples was conducted by 32 semi-trained panelists from Melkassa Agricultural Research
Center, whose ages ranged from 24 to 35. The samples were tested following Zewdu et al.26 procedures for softness, stickiness, rollability, sourness, bitterness, color, odor, flavor, injera eyes and overall sensory acceptability parameters using a seven-point hedonic scale with the criteria: 1 is dislike extremely and 7 is like extremely.
Statistical analysis and optimization: To establish the level of significance within means, Duncan’s multiple range t-test (IBM SPSS statistical software package, version 23.0) was used. In each response, the reliability of the terms in the regression equations was tested using analysis of variance, with the significance test level set at 5% (p<0.05).
Design-Expert was used to produce formulation tests, best-fit regression models of responses, build contour plots, response surface plots and overlaid plots and optimize the results. Using the Design-Expert software (version 13.0.5.0), both numerical and graphical optimization techniques were used with a requirement of the lowest possible teff while sorghum and fenugreek were left in ranges.
RESULTS AND DISCUSSION
Proximate composition of the formulated injera: The moisture, total ash, crude fiber, crude fat, crude protein, carbohydrate content and energy of all formulated injera are displayed in Table 2. Except for moisture content, all properties are stated on a dry basis (% DM) while moisture is expressed on a wet basis (Wb).
Protein content: Sorghum, teff and fenugreek had a significant effect on the final protein content. The average protein level of all injera varied from 11.37 to 17.19% (Table 2) which was in close agreement with the findings of Lamesgen et al.27, who obtained a range of 11.78 to 18.84% using a composite lupine flour with teff flour in injera. The injera product with 50% teff, 45% sorghum and 5% fenugreek blended had the highest relative crude protein level, whereas the 100% teff injera without any sorghum and fenugreek mix had the lowest relative crude protein content. Injera made from composite flours containing a high ratio of fenugreek has a high protein content. The relationship of the proportion of flour ingredients to the protein content of the formulated injera (Fig. 1). The increase in protein content observed may be due to the blended flours improving or optimizing the protein content of products4,26. In addition, this finding may be due to the synthesis of enzymes or compositional proportion changes followed by the degradation of the components.
| Table 2: | Proximate composition for injera formulations | |||
| Proximate composition of injera | |||||||
| Moisture | Ash | C. Fat | C. Fiber | C. Protein | U. CHO | Energy | |
| Formulation | (%Wb) | (%DM) | (%DM) | (%DM) | (%DM) | (%DM) | (kcal/100 g) |
| T1 | 66.44±0.113cd | 3.06±0.014c | 10.34±0.001b | 4.30±0.001ef | 14.73±0.041c | 62.72±0.007i | 385.62±0.495a |
| T2 | 60.04±0.177h | 3.14±0. 014b | 2.50±0.001h | 4.18±0.000f | 11.36±0.014g | 77.71±0.014a | 362.05±0.007f |
| T3 | 62.19±0.127f | 2.92±0. 014e | 2.58±0.000h | 5.38±0.003bcd | 11.71±0.014f | 76.91±0.00b | 356.14±0.495g |
| T4 | 61.75±0.354fg | 3.02±0. 014cd | 3.24±0.003g | 4.64±0.001cdef | 12.67±0.014e | 74.84±0.05d | 360.64±0.219f |
| T5 | 61.43±0.608g | 2.92±0. 014e | 2.57±0.001h | 5.39±0.001bcd | 11.73±0.028e | 76.15±0.028c | 357.09±0.014g |
| T6 | 65.99±0.014d | 2.97±0. 014de | 8.66±0.000d | 4.71±0.007cdef | 14.71±0.014c | 65.01±0.014g | 378.00±0.028cd |
| T7 | 63.79±0.014e | 2.78±0. 014f | 3.52±0.000f | 5.54±1.413def | 14.04±0.0028d | 73.07±0014e | 357.96±5.713f |
| T8 | 56.16±0.678i | 3.91±0. 014a | 7.00±0.028e | 5.16±0.003bcde | 14.80±0.424c | 66.29±0.297f | 366.73±2.022e |
| T9 | 67.79±0.339b | 2.76±0. 014f | 8.91±0.001c | 5.50±0.000bc | 15.91±0.014b | 63.56±0.057h | 376.06±0.177d |
| T10 | 66.89±0.198c | 3.05±0.071c | 10.34±0.000b | 4.30±0.003ef | 14.74±0.014c | 62.34±0.00j | 384.21±0.184ab |
| T11 | 69.92±0.028a | 2.64±0. 014g | 10.84±0.000a | 6.00±0.124b | 17.20±0.000a | 59.54±0.014k | 38.79±0.446bc |
| T12 | 59.67±0.014h | 3.15±0.0b | 2.43±0.028 h | 4.18±0.000f | 11.37±0.014g | 77.74±0.014a | 361.59±0.255f |
| T13 | 63.21±0.297e | 2.68±0. 014g | 2.26±0.000i | 6.99±0.004a | 14.08±0.014d | 74.99±0.028d | 348.68±0.149h |
| T14 | 63.76±0.085e | 2.67±0.028g | 2.26±0.004i | 6.99±0.001a | 14.07±0.014d | 74.86±0.064d | 348.10±0.269h |
| Mean | 63.5 | 2.98 | 5.53 | 5.23 | 13.86 | 70.41 | 365.98 |
| CV (%) | 0.61 | 0.21 | 0.44 | 0.024 | 2.04 | 0.43 | 0.18 |
| Values are Mean±Standard Deviation in duplicate runs, values followed by different letters within a column indicate significant differences (p<0.05), DM: Dry matter, CV: Coefficient of variance, C: Crude, U.CHO: Utilizable carbohydrate content and Energy: Gross energy | |||||||
|
The higher level interactions (special cubic effects), teff×sorghum and teff×sorghum×fenugreek amounts had a negative impact on response protein content whereas teff×sorghum and sorghum×fenugreek amounts had a positive impact on response. Each factor had a linear and special cubic effect on the response; this is exemplified by the special cubic model as shown in Fig. 1, which is represented by:
Y =+11.36×A+14.08×B-355.23×C-4.00×AB+444.78×AC+445.05×BC-5.47×ABC |
where, A is teff flour, B is sorghum flour and C is fenugreek flour.
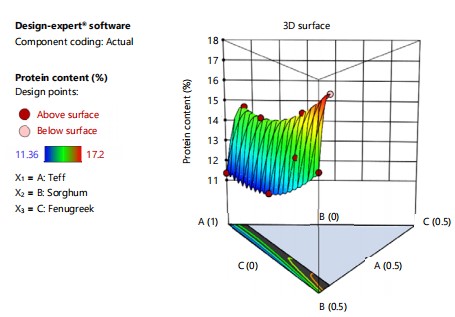
Moisture content: The mean moisture content of the composite injera samples was significantly affected by the blending ratios at p<0.05. The formulated injera’s mean moisture content ranged from 56.16 to 69.92%. The injeras made from 50% teff blended with 45% sorghum and 5% fenugreek had the highest mean moisture content, while the injeras made from 73% teff blended with 24% sorghum and 3% fenugreek had the lowest moisture content (56.16%). This is because raw sorghum and fenugreek have a higher fiber content than teff varieties and fibers tend to absorb water. This shows that the blending ratio affects the softness of injera products and moisture variation at controlled time-temperature baking conditions. In Fig. 2, the moisture content of injera increases as the fenugreek and sorghum blending ratio increases. Previous research by Zewdu et al.26 and Lamesgen et al.27 indicates injera made from various cereals ranges in moisture content between 59.34-66.97%. Moisture content was found to be significantly different in the model and linear mixture (p<0.0001).
Total ash: The product had an average total ash content between 2.64-3.91% dry matter. Injera prepared from composite flours with a larger proportion of 74% teff, 24% sorghum and 3% fenugreek had the highest ash content. This might be explained by the presence of ash in fenugreek seed flour at a higher amount (up to 3.38)28, followed by teff flour (up to 3.16)9 than in sorghum (up to 2.29%)29 and their interaction with one another influences the ash content product. In Table 3 the ash content obtained a highly significant difference in the model and linear mixture (p<0.0001).
|
| Table 3: | Coefficient of determination (R2), adjusted R² and model significance for proximate composition of injera samples | |||
| Source | Moisture | Ash | Fat | Fiber | Protein | Carbohydrate | Energy |
| Model | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| (1)Linear mixture | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| AB | 0.6657 | 0.0941 | 0.0005 | <0.0001 | <0.0001 | 0.4463 | 0.0564 |
| AC | <0.0001 | <0.0001 | 0.0009 | <0.0001 | <0.0001 | 0.0092 | 0.0116 |
| BC | <0.0001 | <0.0001 | 0.0008 | <0.0001 | <0.0001 | 0.0093 | 0.0137 |
| ABC | 0.0008 | <0.0001 | 0.0010 | 0.0128 | |||
| AB (A-B) | 0.0129 | <0.0001 | 0.2054 | ||||
| AC (A-C) | 0.0011 | <0.0001 | 0.0104 | ||||
| BC (B-C) | 0.0006 | <0.0001 | 0.0184 | ||||
| A2BC | <0.0001 | <0.0001 | 0.0102 | ||||
| AB2C | <0.0001 | <0.0001 | 0.0093 | ||||
| ABC2 | <0.0001 | <0.0001 | 0.0115 | ||||
| Lack of fit | 0.799 | 0.6956 | 0.5857 | 0.5443 | |||
| R2 | 0.9965 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 0.9993 | 0.9992 |
| Adjusted R2 | 0.9908 | 0.9997 | 1.0000 | 1.0000 | 1.000 | 0.9958 | 0.9973 |
| A: Teff, B: Sorghum and C: Fenugreek | |||||||
Crude fiber content: The crude fiber content of the composite injera was significantly affected by the blended products (p<0.05). The baked injera’s crude fiber content ranged from 4.179 to 6.988%. The highest fiber content (6.988%) was found in injera made from 50% teff and 50% sorghum with no fenugreek, while the lowest crude fiber content was found in injera made entirely from teff (4.179%). However, Ashenafi30 found that injera made from different cereals had crude fiber content varying from 0.8-5.2% DM. The crude fiber content of formulated injera differed from previous studies, possibly due to variations in composition amount and variety used. In Fig. 3 the model graphs that the proportion of sorghum and fenugreek flour in injera increased in tandem with the increase in crude fiber content. Crude fiber content demonstrated a highly significant difference in the cubic model and linear mixture (p<0.0001). The predictive model equation obtained for crude fiber content (Yc):
Yc = +4.18×A+6.99×B-8499.79×C-0.7930×AB+14137.28×AC+12474.18×BC-9718.62× ABC+20.87×AB×(A-B)-5858.90×AC×(A-C)-3792.58×BC×(B-C)R2 = 1.00 |

|
Utilizable carbohydrate content:
The baked injera’s carbohydrate content ranged from 59.54 to 77.74%. Carbohydrate content in injera made from various combination flours ranged from 73.89 to 86.8%18,26,31. The CHO of formulated injera differed from previous studies, possibly due to variations in composition amount and the variety used. Other factors that contributed to CHO content such as fermentation temperature and the relatively higher content of protein, crude fat content and total ash contributed relatively less than the previous studies.
Injera made from 100% teff had a significantly higher amount of utilizable carbohydrate (CHO) (77.74%) while the lowest CHO content (59.54%) of injera was made from 50% teff, 45% sorghum and 5% fenugreek. This could be because the carbohydrate content of raw fenugreek, followed by sorghum, was lower than that of the teff flour sample. Each computed mean blending ratio injera carbohydrate content showed a significant variation (p<0.05) and the formulated composite ratio injera CHO demonstrated a highly significant difference (p<0.0001) in the cubic and linear mixture models of the interaction composite flours.
Crude fat content: The crude fat content of injera products showed significant differences (p<0.05). Injera blends crude fat content ranged from 2.26 to 10.838%. The highest crude fat content was obtained by blending 50% teff, 45% sorghum and 5% fenugreek and the lowest crude fat content was recorded as 50% teff and 50% sorghum injera. The crude fat content of injera increased as the proportion of fenugreek ingredients increased. These findings were consistent with injera-containing fenugreek flour had a higher crude fat content than injera made entirely of teff flour16.
Gross energy: Blended injera had a gross energy content ranging from 348.10 to 385.62 kcal/100 g. The highest gross energy values were obtained when 95% teff injera was blended with 5% fenugreek, while the lowest value was obtained when 50% teff and 50% sorghum injera were used alone. As a result, the energy contents in the blended injera sample (T1, T6, T8 T9, T10 and T11) appeared to be higher.
| Table 4: | Antinutritional factor and mineral contents for raw and injera samples | |||
| Antinutritional Factors (mg/100 g DM) | Minerals (mg/100 g DM) of Injera | ||||
| Sample | Condensed tannin | Phytic acid | Iron | Zinc | Calcium |
| Raw material | |||||
| Teff | 15.987±0.2 | 972.258±0.06 | 18.573±0.08 | 3.733±0.31 | 149.493±0.2 |
| Sorghum | 1.479±0.3 | 79.21±0.18 | 3.983±0.012 | 1.523±0.21 | 5.634±0.1 |
| Fenugreek | 103.08±0.17 | 73.89±0.10 | 27.941±0.13 | 2.402±0.02 | 221±0.41 |
| Formulation | |||||
| T1 | 4.11±0.030a | 174.84±0.042a | 21.21±0.028a | 2.31±0.014a | 151.13±0.028a |
| T2 | 0.89±0.565g | 132.93±0.014d | 20.13±0.014b | 2.36±0.014a | 146.88 + 0.000b |
| T3 | 0.67±0.000fg | 102.64±0.000i | 16.48±0.000f | 1.86±0.014c | 111.37±0.000f |
| T4 | 1.23±0.001e | 126.36±0.028f | 18.59±0.000d | 2.13±0.028b | 130.65±0.014d |
| T5 | 0.67±0.000fg | 102.42±0.000j | 16.49±0.014f | 1.85±0.014c | 111.34±0.000f |
| T6 | 2.76±0.001b | 152.03±0.000b | 19.24±0.014c | 2.10±0.014b | 133.16±0.028c |
| T7 | 1.01±0.000ef | 96.82±0.000k | 14.94±0.000h | 1.59±0.000d | 95.13±0.014h |
| T8 | 2.02±0.001d | 130.59±0.000e | 17.47±0.028e | 1.89±0.000c | 116.74±0.028e |
| T9 | 2.33±0.001c | 126.14±0.028g | 16.34±0.014g | 1.63±0.000d | 101.93±0.000g |
| T10 | 4.11±0.000a | 174.80±0.000a | 21.23±0.000a | 2.30±0.000a | 151.10±0.141a |
| T11 | 2.71±0.000b | 121.56±0.014h | 14.63±0.014i | 1.33±0.028e | 87.11±0.000i |
| T12 | 0.89±0.000ef | 133.03±0.000c | 20.11±0.000b | 2.36±0.014a | 146.86±0.042b |
| T13 | 0.45±0.001g | 72.83±0.028l | 12.84±0.014j | 1.19±0.000f | 75.28±0.000j |
| T14 | 0.45±0.001g | 72.84±0.028l | 12.83±0.014j | 1.20±0.000f | 75.26±0.028j |
| Mean | 1.74 | 122.85 | 17.32 | 1.86 | 116.71 |
| CV (%) | 0.068 | 0.071 | 0.065 | 0.33 | 0.015 |
| Values are Mean±Standard Deviation in duplicate runs and values followed by different letters within a column indicate significant differences (p<0.05) | |||||
Multiple regression for gross energy suggested that the addition of fenugreek resulted in the highest gross energy followed by teff and sorghum, respectively. The interaction effect of injera made from×B, A×C, B×C×A×B×C, A×C(A-C), B×C×(B-C), A²×B×C, AB²C and ABC² was shown to be highly significant at p<0.5, but interaction effect of injera made from A×B×(A-B) was not significant difference at p<0.05. The cubic regression model for gross injera energy (Yge):
Yge = 361.82×A+348.39×B+(2.033×105)×C+6.04×AB-(3.203×105)×AC-(3.083×105)× BC+(2.252×105)×ABC-151.29×AB×(A-B)+(1.188×105)×AC×(A-C)+(1.040×105)×BC (B-C)R2 = 0.9992 |
Antinutritional factors, the total mineral content of injera: Table 4 shows the antinutritional factors and minerals content of the injera formulations.
Mineral content of injera: Among the interaction and blended component mixtures, teff-fenugreek resulted in the greatest increase in total iron content followed by teff injera. The combination of sorghum and teff lowered the iron level of the injera. The iron content of the composite injera ranges from 12.83 to 21.21 mg/100 g. The iron content of injera processed from teff-fenugreek without sorghum is significantly (p<0.05) higher (21.21 mg/100 g) than the injera processed from teff-sorghum composite because fenugreek has iron contents (up to 33.5 mg/100 g)32 higher than teff grain iron content (25.13 mg/100 g) whereas sorghum has a lower iron content (4.1 mg/100 g)33. A minimum value (21.21 mg/100 g) was obtained at 95% teff, 5% fenugreek and 0% sorghum. Due to the lower iron content of sorghum grain, the iron content of injera decreased as the percentage of sorghum increased. The iron content was found to be significantly different (p<0.05) in linear and cubic models. The Fe content regression equation Y (Fe):
Y (Fe) =+20.12×A+12.84×B-2616.84×C+0.0300×AB+5259.25×AC+3005.54×BC-2989.56×ABC+25.40× AB (A-B)-2896.34 AC (A-C)-79.67×BC (B-C)R2 = 1.0000 |
The total zinc content ranged from 1.19 to 2.36 mg/100 g. Injera prepared from pure teff had significantly (p<0.05) higher zinc content (2.36 mg/100 g) than other formulations. The lowest values (1.19 mg/100 g) were recorded in injera comprising 50% teff and 50% sorghum. This is explained by the higher content of zinc (2.4-6.8 mg/100 g) in teff flour than sorghum (1.4-1.7 mg/100 g)33 and fenugreek (up to 2.5 mg/100 g)32. Table 5 shows the interaction of injera made from composite flours of BC, A×B×C, AB×(A-B), A×C×(A-C) and A×B, B×C and B×C×(B-C) were not statistically significant (p>0.05) whereas A×B was significantly different at p<0.05. The regression model obtained for zinc content was:
| Table 5: | Coefficient of determination (R2), adjusted R² and model significance for mineral content and antinutritional factors of injera samples | |||
| Source | Condensed tannin | Phytic acid | Iron | Zinc | Calcium |
| Model | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 1Linear mixture | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| AB | 1.0000 | 0.0073 | 0.4818 | 0.0001 | <0.0001 |
| AC | 0.0003 | 0.0003 | 0.0132 | 0.1465 | <0.0001 |
| BC | 0.0058 | 0.0003 | 0.0744 | 0.127 | <0.0001 |
| ABC | 0.0008 | 0.0003 | 0.029 | 0.1394 | <0.0001 |
| AB (A-B) | <0.0001 | 0.4214 | 0.0001 | 0.2397 | 0.0457 |
| AC (A-C) | <0.0001 | 0.0003 | 0.0029 | 0.1882 | <0.0001 |
| BC (B-C) | 0.0623 | 0.0003 | 0.8714 | 0.115 | <0.0001 |
| R² | 1.0000 | 1.0000 | 1.0000 | 0.9999 | 1.0000 |
| Adjusted R² | 1.0000 | 1.0000 | 1.0000 | 0.9998 | 1.0000 |
| A: Teff, B: Sorghum and C: Fenugreek | |||||
2.36×A+1.20×B-818.52×C+0.3100×AB+1220.41×AC+1318.70×BC-904.73×ABC-1.29×AB×(A-B)- 386.16×AC×(A-C)-508.01×BC×(B-C)R2 = 0.9999 |
All composite injera had varying calcium levels (75.26-151.13 mg/100 g). The sample processed from 95% teff, 0% sorghum and 5% fenugreek had the greatest value (151.26 mg/100 g), while blending 50% teff, 50% sorghum and 0% fenugreek produced the lowest value (75.26 mg/100 g). As the percentage of teff and fenugreek in the mixture grew, so did the calcium level of the injera. The observed high calcium content may be attributed to the higher calcium content of teff (165.2 mg/100 g)3 and fenugreek (176 mg/100 g)32 than that of sorghum (5.0-5.8 mg/100 g)33. The predictive model obtained for calcium content was:
+146.87×A+75.27×B-79970.12×C+1.14×AB+1.243×105×AC+1.249×105×BC-89724.82 ABC-7.89×AB×(A-B)-44040.18×AC×(A-C)-44771.34×BC×(B-C)R2 = 1.0000 |
Anti-nutrient content of injera: The interaction of teff, sorghum, fenugreek flour and blending ratios affected the condensed tannin significantly (p<0.05). The content of condensed tannins ranges from 0.45 to 4.11 mg/100 g (Table 4). The lowest values (0.45 mg/100 g) were found in injera made with 50% teff, 50% sorghum and 0% fenugreek, while the highest was 4.11 mg/100 g in injera made with 95% teff and 5% fenugreek. This could also be due to the effect of heat on tannin causing some form of interaction with other grain components, especially with protein-forming complexes that will not be extractable to determine using the method of analysis34. The linear mixture and cubic model were significantly different (p<0.0001). The interaction injera made from composite flours except A×B, all the interaction effects of AC, BC, A×B×C, AB×(A-B), A×C×(A-C) and B×C×(B-C) show significant differences at p<0.05.
As shown in Table 4 the phytate (phytic acid) content of teff-sorghum-fenugreek injeras was significantly affected by the interaction of varieties and blending ratios (p<0.05) and there was a significant difference (p<0.05) between each blending ratio and controls with phytate content in the product. The teff blend with 95% teff, 0% sorghum and 5% fenugreek had the highest phytic acid content in the composite injera. The 50% teff and 50% fenugreek blend had the lowest value. The phytic acid content of the blends had lower values in blended teff-sorghum flours than with teff-fenugreek flours. The predicted model also showed that the phytic acid content of composite flour was influenced by fenugreek flour>teff flour>sorghum flour. Studies on the spontaneous fermentation of teff dough showed different magnitudes of phytic acid degradation in the range of 42-80%35. This fermentation process has the capacity to reduce phytic acid in the preparation of teff injera.
The interaction injera made from composite flours of AC, BC, A×B×C, A×C×(A-C) and B×C×(B-C) demonstrated significant difference at p<0.05, but AB×(A-B) showed non-significant difference at p<0.05.
Alkaline water retention capacity and staling rate of the formulated injera
Effect of formulation ingredients on the alkaline water retention capacity of injera: Alkaline water retention capacity (AWRC) of the formulated injera was taken as an indication of staling degree and freshness. Table 6 shows at zero time and after 24, 48 and 72 hrs of storage, the alkaline water retention capacity, AWRC (%) value for the composite flour injera, varied from 49.42 to 84.0, 32.92 to 76.49, 32.64 to 76.01 and 23.57 to 73.66, respectively. The AWRC of the formulated injera at 0, 24 and 48 hrs demonstrated a significant shift in the responses for linear mixture and component model terms at (p<0.0001). The interaction effect of mixed flour proportions of injera made from A×B, A×C, B×C, A×B×C, A×B×(A-B), A×C×(A-C) and B×C×(B-C) of alkaline retention capacity at zero time and after 48 hrs and A×B of alkaline retention capacity after 72 hrs showed extremely significant difference at (p<0.05), but A×C and B×C after 72 hrs demonstrated non-significant difference at p<0.05.
The addition of fenugreek to the various sorghum-teff injera formulations increased AWRC when compared to the control (teff alone) injera and teff-sorghum blend injera, owing to fenugreek’s higher fiber content. When compared to the zero-time period, the freshness of all formulated injera blends and the injera control decreased over time. Furthermore, during storage at room temperature for 24, 48 and 72 hrs, the rate of decrease of freshness of all blends in formulated injera was reduced. This effect is attributed to fiber’s well-known water binding capacity, which prevents water loss during storage, as well as a possible interaction between fiber and starch, which slows starch retrogradation36.
Effect of formulation ingredients on the response percentage of staling: After 24, 48 and 72 hrs of storage, the staling rate values for the teff injera alone (control) and injera made from composite flours of teff, sorghum and fenugreek were determined to be in the ranges of 8.95-33.43, 9.52-34.58 and 12.32-52.31%, respectively. The staling rate (SR) of formulated injera stored as mentioned above for the linear mixture and model interaction of components exhibited a highly significant difference at (p<0.0001).
| Table 6: | Alkaline water retention capacities and staling rates for injera samples | |||
| Alkaline water retention capacity and staling rate | |||||||
| Formulation | AWRC (%) zero time |
AWRC (%) after 24 hrs |
SR (%) after 24 hrs |
AWRC (%) after 48 hrs |
SR (%) after 48 hrs |
AWRC (%) after 72 hrs |
SR (%) after 72 hrs |
| T1 | 78.68±0.210b | 71.11±0.231b | 9.62±0.2b | 68.25±0.1b | 13.26±0.12j | 64.57±0.120b | 17.93±0.221h |
| T2 | 52.09±0.211i | 39.83±0.131h | 23.54±0.152b | 38.93±0.311i | 25.26±0.251d | 31.07±0.121f | 40.35±0.181e |
| T3 | 53.75±0.140h | 41.09±0.090g | 23.55±0.121b | 40.65±0.212h | 24.37±0.180e | 31.22±0.160f | 41.92±0.300c |
| T4 | 58.28±0.061g | 41.09±0.150g | 21.33±0.111b | 43.22±0.231f | 25.84±0.152c | 34.46±0.211ef | 40.87±0.131e |
| T5 | 53.61±0.163h | 41.13±0.132g | 23.28±0.292b | 40.83±0.241h | 23.84±0.211f | 31.26±0.321h | 41.69±0.240c |
| T6 | 67.08±0.441d | 53.44±0.232d | 20.33±0.322b | 52.61±0.412d | 21.57±0.390i | 45.7±0.280cd | 31.87±0.361g |
| T7 | 60.87±0.160f | 46.43±0.241f | 23.72±0.213b | 46.28±0.513f | 23.97±0.321f | 35.69±0.411ef | 41.37±0.272c |
| T8 | 62.62±0.331e | 48.43±0.290e | 22.66±0.312b | 47.99±0.351e | 23.36±0.340g | 39.41±0.171de | 37.06±0.251f |
| T9 | 72.93±0.312c | 55.88±0.241c | 23.39±0.221b | 56.93±0.342c | 21.94±0.330h | 49.86±0.180c | 31.63±0.241g |
| T10 | 78.72±0.212b | 71.05±0.231b | 9.74±0.221b | 68.96±0.1b | 12.40±0.001k | 64.86±0.120b | 17.61±0.221i |
| T11 | 84.01±0.421a | 76.49±0.362a | 8.95±0.381b | 76.01±0.282a | 9.52±0.351l | 73.66±0.271a | 12.32±0.342g |
| T12 | 52.11±0.191i | 39.99±0.121h | 23.26±0.142b | 39.03±0.301i | 25.10±0.243d | 32.74±0.110ef | 37.17±0.171f |
| T13 | 49.42±0.163j | 32.92±0.082j | 33.39±0.121b | 32.64±0.152j | 33.95±0.172b | 23.57±0.091gh | 52.31±0.131a |
| T14 | 49.92±0.141j | 33.23±0.100i | 33.43±0.121b | 32.66±0.132j | 34.58±0.141a | 24.38±0.061gh | 51.16±0.102b |
| Mean | 62.44 | 49.44 | 21.44 | 48.93 | 22.78 | 41.6 | 35.38 |
| CV (%) | 0.3 | 0.25 | 0.67 | 0.53 | 1.86 | 1.6 | 4.38 |
| Values are Mean±Standard Deviation in duplicate runs. Values followed by different letters within a column indicate significant differences (p<0.05) | |||||||
| Table 7: | Sensory acceptability test result of the mean formulated fresh injera samples using 7-point hedonic scale | |||
| Formulation | Sourness | Bitterness | Softness | Stickiness | Roll ability | Odor |
| T1 | 5.57±0.156bc | 6.29±0.41a | 6.14±0.113abc | 5.64±0.240abc | 5.93±0.042b | 5.71±0.849bc |
| T2 | 6.37±0.099a | 5.86±0.085ab | 6.19±0.256abc | 5.88±0.000ab | 6.24±0.325ab | 5.71±0.014bc |
| T3 | 5.71±0.127bc | 5.87±0.028ab | 6.34±0.113abc | 5.86±0.071abc | 6.29±0.113ab | 5.57±0.141c |
| T4 | 5.57±0.141bc | 5.71±0.268ab | 5.93±0.028abc | 5.86±0.156abc | 6.29±0.071ab | 5.57±0.141c |
| T5 | 5.57±0.141bc | 6.29±0.014a | 6.57±0.028a | 6.43±0.141a | 6.57±0.255b | 5.93±0.042bc |
| T6 | 5.64±0.566bc | 5.29±0.569b | 5.14±0.170d | 5.14±0.184c | 6.00±0.283b | 6.29±0.127ab |
| T7 | 5.57±0.156bc | 5.93±0.042ab | 6.14±0.198abc | 5.71±0.976abc | 6.00±0.141ab | 5.86±0.085bc |
| T8 | 6.00±0.141ab | 6.00±0.848ab | 5.86±0.057bc | 6.00±0.141ab | 6.43±0.028ab | 6.57±0.028a |
| T9 | 6.00±0.424ab | 5.86±0.071ab | 6.43±0.042ab | 6.29±0.000ab | 6.29±0.156ab | 6.57±0.141a |
| T10 | 5.29±0.057cd | 5.83±0.042ab | 5.71±0.021d | 5.71±0.014abc | 6.00±0.424b | 5.57±0.156c |
| T11 | 5.00±0.141d | 5.43±0.042b | 5.86±0.099bc | 6.29±0.283ab | 6.43±0.042ab | 5.71±0.156bc |
| T12 | 6.29±0.255a | 6.00±0.566ab | 6.43±0.255ab | 6.29±0.014ab | 6.14±0.127ab | 5.71±0.000bc |
| T13 | 5.40±0.283cd | 5.24±0.283b | 5.79±0.141bc | 6.00±0.283ab | 6.27±0.099ab | 6.10±0.000abc |
| T14 | 5.86±0.071b | 5.43±0.141b | 6.00±0.849abc | 6.14±0.099ab | 6.23±0.297ab | 6.29±0.141ab |
| Mean | 5.7 | 5.79 | 6.04 | 5.95 | 6.22 | 5.94 |
| CV (%) | 4.15 | 4.07 | 3.41 | 4.28 | 1.75 | 2.69 |
| Formulation | Flavor | Eye distribution | Color | Overall acceptability | pH | |
| T1 | 6.64±0.184a | 5.86±0.085bc | 5.00±0.283cd | 5.86±0. 219ab | 3.83±0.014cd | |
| T2 | 5.71±0.028def | 6.14±0.170ab | 5.84±0.042b | 5.99±0. 047ab | 3.82±0.001cd | |
| T3 | 5.81±0.127cde | 5.86±0.028bc | 5.86±0.028b | 5.91±0. 083ab | 3.98±0.014b | |
| T4 | 5.43±0.028f | 5.64±0.127c | 5.00±0.000cd | 5.67±0. 024ab | 4.08±0.014a | |
| T5 | 6.14±0.085bc | 6.43±0.269a | 6.00±0.000ab | 6.21±0. 086a | 3.98±0.000b | |
| T6 | 6.07±0.099bc | 6.29±0.099a | 5.29±0.127c | 5.68±0. 778ab | 4.11±0.000a | |
| T7 | 5.57±0.0.014ef | 5.57±0.057c | 5.14±0.042cd | 5.72±0. 016ab | 3.98±0.003b | |
| T8 | 6.71±0.042a | 6.43±0.113a | 5.86±0.085b | 6.21±0. 125a | 3.86±0.014c | |
| T9 | 6.14±0.084bc | 6.43±0.028a | 6.00±0.141ab | 6.22±0. 089a | 3.67±0.000e | |
| T10 | 6.27±0.099b | 5.49±0.297c | 4.87±0.085d | 5.64±0. 124b | 3.81±0.014cd | |
| T11 | 5.86±0.141cde | 6.29±0.127a | 6.14±0.113ab | 5.89±0. 026ab | 3.75±0.000de | |
| T12 | 5.43±0.000f | 6.29±0.000a | 5.86±0.156b | 6.05±0. 560ab | 3.82±0.014cd | |
| T13 | 5.91±0.000cde | 5.81±0.000bc | 6.14±0.085ab | 5.85±0. 447ab | 3.80±0.014cd | |
| T14 | 6.04±0.057bcd | 6.29±0.283a | 6.29±0.269a | 6.06±0. 147ab | 3.80±0.000cd | |
| Mean | 5.98 | 6.06 | 5.66 | 5.93 | 3.88 | |
| CV (%) | 3.45 | 4.39 | 1.52 | 2.58 | 0.15 | |
| Values are Mean±Standard Deviation in duplicate runs and values followed by different letters within a column indicate significant differences (p<0.05) | ||||||
Fenugreek inclusion in the formulation delayed the staling compared to the control after 0, 24, 48 and 72 hrs of storage as detailed in Table 6. The higher fat and protein content of formulated injera could explain these findings. These findings concurred with the previous studies by Calle et al.37, who concluded that the high fat and protein content contributed to the reduced staling rate. Multiple regression for staling generally indicated that an increase in the level of fenugreek produced a decrease in the rate of staling, while sorghum produced the highest increase in staling rate, followed by teff in response to formulated injera.
Effect of blending ratios on sensory acceptability and pH of injera: The color of the baked injera ranged from 4.87 to 6.29. The influence of varied quantities of fenugreek seed flour on color acceptance evaluation of fenugreek flour replacement teff injera samples was found to follow a similar pattern as observed in study16. The interaction effect of injera made from AC, BC, ABC, AC(A-C) and BC(B-C) was shown to be highly significant at p<0.5, but the interaction effect of injera made from AB and AB(A-B) was not significant at p<0.5.
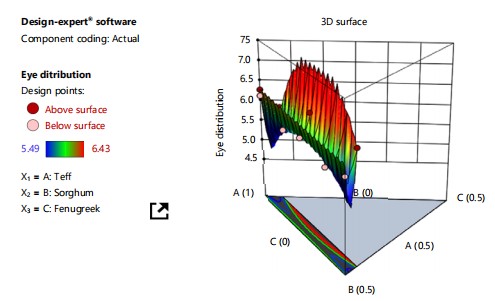
The eye distribution result of all the injera products ranged from 5.49 to 6.43. At p<0.05, the analysis of variance blending ratios had no significant impact on the eye distribution result of all formulated injera. According to a predictive model for eye distribution, the addition of teff resulted in the highest hedonic ratings for eye distribution, followed by sorghum and fenugreek as shown in Fig. 4. Table 7 shows the lowest panelist scores of the injera eyes were recorded at 95% teff and 5% fenugreek blends. The blending proportion of ingredients and their interaction might have a positive effect or might reduce the injera’s eye appearance score when compared to injera prepared solely with teff, as determined by the panelists.
|
This could be because fermentation produces less carbon dioxide. The eyes of injera at the top surface are produced during cooking due to the release of CO2 bubbles. Sorghum injera has low sensory quality and fewer gas holes on the surface, according to Fox et al.5.
The analysis of variance at p<0.05 showed blending ratios had a significant impact on the flavor result of all formulated injera, with the flavor result ranging from 5.43 to 6.71. The linear mixture and special quartic models demonstrated an interaction of composition ingredients and ANOVA interaction between flavor acceptance and injera mixing ratio was substantially different at p<0.05. The analysis of variance showed the interaction effect of injera made from the blending flour ratios of A×C, B×C, A²×B×C, A×B²×C and A×B×C² was significant at p<0.05, whereas A×B was not significantly different at p<0.05.
The odor variance analysis of all formulated injera had a significant impact on the linear mixture and special quartic models at (p<0.05). The T8 and T9 injera, which were produced with (74% teff, 24% sorghum and 3% fenugreek) and (62% teff, 34% sorghum and 4% fenugreek) respectively, were preferred by the panelists. According to multiple regression for taste, the addition of sorghum resulted in the greatest hedonic scores for odor, followed by teff and fenugreek. This could be due to taste molecules being generated by lactic acid bacteria. Fermentation increases the flavor of food38.
The formulated injera’s roll ability and its interaction effect were not substantially different at (p<0.05). The baked injera roll ability score ranged from 5.93 to 6.57, according to the panelists. The T5 (75% teff and 25% sorghum) had the highest roll ability score, whereas T1 (95% teff, 0% sorghum and 5% fenugreek) had the lowest. The roll ability of the fenugreek replaced injera was reduced as the amount of fenugreek flour in the injera substitution increased. This could be related to the gelatinization capability of raw material, which affects injera’s ability to roll39.
The formulated injera’s softness and its interaction impact were not substantially different at (p<0.05). Roll ability is one criterion used in Ethiopia to evaluate injera quality; premium injera could be rolled without breaking or sticking. The baked injera roll ability score according to the panelists, ranged from 5.14-6.57.
| Table 8: | Coefficient of determination (R2), adjusted R² and model significance for sensory acceptability of injera samples | |||
| Source | Sourness | Bitterness | Softness | Stickiness | Roll ability |
Odor | Flavor | Eye distribution |
Color | OAA | pH |
| Model | 0.03 | 0.1226 | 0.0332 | 0.2407 | 0.1014 | 0.009 | 0.0254 | 0.1247 | 0.0011 | 0.148 | <0.0001 |
| 1Linear mixture |
0.0162 | 0.1011 | 0.0606 | 0.2111 | 0.0897 | 0.0156 | 0.0192 | 0.7033 | 0.0004 | 0.2892 | <0.0001 |
| AB | 0.0845 | 0.0691 | 0.0785 | 0.7745 | 0.09 | 0.1469 | 0.262 | 0.9809 | 0.2415 | 0.2179 | <0.0001 |
| AC | 0.266 | 0.3018 | 0.6154 | 0.3094 | 0.0703 | 0.0787 | 0.0066 | 0.0387 | 0.0038 | 0.0464 | <0.0001 |
| BC | 0.2676 | 0.3006 | 0.621 | 0.4265 | 0.093 | 0.0773 | 0.0065 | 0.0414 | 0.0046 | 0.0467 | 0.0002 |
| ABC | 0.3612 | 0.0798 | 0.0184 | 0.0041 | <0.0001 | ||||||
| AB (A-B) | 0.0897 | 0.1143 | 0.1193 | <0.0001 | |||||||
| AC (A-C) | 0.2088 | 0.0522 | 0.0032 | <0.0001 | |||||||
| BC (B-C) | 0.6183 | 0.1367 | 0.006 | 0.0008 | |||||||
| A²BC | 0.3496 | 0.4685 | 0.6609 | 0.0836 | 0.0095 | 0.0908 | |||||
| AB²C | 0.1697 | 0.1996 | 0.498 | 0.0592 | 0.0074 | 0.0507 | |||||
| ABC² | 0.4079 | 0.258 | 0.8546 | 0.1718 | 0.0103 | 0.0924 | |||||
| Lack of fit | 0.6567 | 0.5105 | 0.9174 | 0.9249 | 0.9088 | 0.7335 | 0.6065 | ||||
| R² | 0.9083 | 0.8262 | 0.9042 | 0.8282 | 0.8977 | 0.9451 | 0.9148 | 0.6847 | 0.9906 | 0.8098 | 0.09993 |
| Adjusted R² | 0.7616 | 0.5484 | 0.7509 | 0.4418 | 0.6677 | 0.8574 | 0.7785 | 0.4145 | 0.9693 | 0.5054 | 0.9979 |
| NB: A: Teff, B: Sorghum and C: Fenugreek | |||||||||||
The T6 (84% teff, 12% sorghum and 4% fenugreek) had the lowest softness score, while the best was obtained at 75% teff and 25% sorghum. Fox et al.5 observed that teff injera is softer which is a sign of higher quality injera than sorghum injera and this is consistent with their findings.
The blending ingredients had a substantial impact on the taste of injera (p<0.05). The control injera had the highest sourness acceptance, while 50% teff, 45% sorghum and 5% fenugreek injera had the lowest sourness acceptance. The acceptance of sourness reduced as the level of fenugreek substitution increased. This could be due to the fact that fenugreek has stronger antinutritive properties than teff and sorghum. Fenugreek has a harsh taste due to the presence of saponins (anti-nutritional elements), which limit its palatability in dishes40.
The baked injera bitterness score, according to the panelists, ranged from 5.24-6.29. The injera made from 50% teff and 50% sorghum had the lowest level of bitterness acceptance, whereas injera made from 95% teff and 5% fenugreek had the highest. As presented in Table 8 the analysis of variance for bitterness shows no significant difference at p<0.05 in the blending ratios and model items.
Ratings for the formulated injera’s overall acceptability ranged from 5.64 to 6.22. On a 7-point hedonic scale, each composition's response was rated as acceptable if it scored higher than 5. Every mix got a mean rating that was significantly higher than normal, indicating a higher-quality product.
The pH is a physicochemical parameter that determines the sourness of injera. The current investigation found that the pH ranged from 3.67 to 4.11. Injera produced from 62% teff, 34% sorghum and 4% fenugreek had the lowest pH value, whereas injera made from 84% teff, 12% sorghum and 4% fenugreek flour had the highest pH. The samples were acidic, possibly due to the higher concentration of fermentable carbohydrates in the formed ingredients and sufficiently acidic to eliminate spoiling bacteria. According to Girma et al.4, the pH of injera ranges from 3.4 to 4.49, which is consistent with the current study. The blending interaction and the model items were shown at p<0.05.
Optimal mixture compositions: The processes were optimized for the response using the derived regression model equation linking the dependent and independent variables. In order to achieve an optimum flour ratio formulation that produces higher injera quality, restrictions on sorghum (0-50%), teff (50-100%) and fenugreek (0-5%) were put in place.
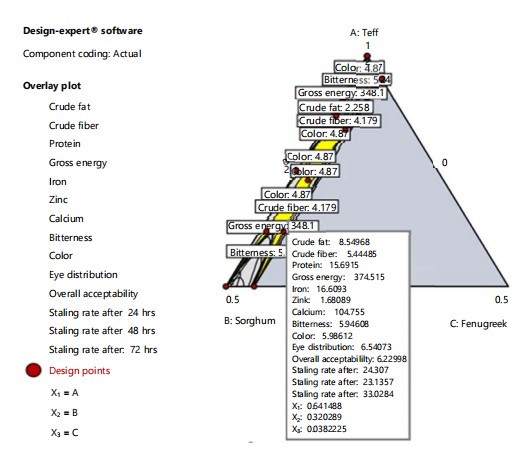
|
When performing general numerical optimization, the maximum bitterness, color, eye distribution, overall acceptability, Fe, Zn, Ca, protein content, crude fiber, crude fat and gross energy responses were given top priority while the staling rate was minimized with the high relative importance of “5” assigned for protein content, gross energy, Fe, Zc, Ca and overall acceptability, whereas the relative importance of “3” was assigned to the others. This is because bitterness, appearance (eye distribution and color) and overall acceptability influence consumer or panelist attitudes toward a product39,41. Protein-energy malnutrition, Fe, Ca, Zn and staling are all common problems1,42. The optimum factor variable levels for teff, sorghum and fenugreek, according to numerical optimization were: 64.1, 32.0 and 3.8% with desirability and 0.590, respectively (Fig. 5). Among these, the optimum predicted response values obtained for this developed injera for crude fat, crude fiber, crude protein, gross energy, Fe, Zn, Ca were 8.549, 5.445, 15.691%, 374.505 kcal/100 g, 16.610, 1.681 and 104.761 mg/100 g, respectively (Fig. 5). The Fig. 5 shows the optimization performed indicated that injera produced from 64.1% teff, 32% sorghum and 3.8% fenugreek had predicted response values for bitterness, color, eye distribution, overall acceptability, were 5.946, 5.987, 6.540, 6.230 and staling rate after 24, 48 and 72 hrs was 24.309, 23.140 and 33.032%.
CONCLUSION
An efficient approach based on mathematical modeling was employed in this work to determine blending ratios for injera preparation with optimal injera quality features. Each element had an important influence on the quality of injera. These flour blending ratios contributed to maximizing the quality attribute of injera. Optimized formulation was predicted according to the polynomial models generated by the design. The D-optimal mixture expert design was successfully applied to find the best combination of teff, sorghum and fenugreek for injera production. In addition, fenugreek inclusion in the formulation delayed the staling and may have enhanced overall sensory acceptability. This could ultimately help to avail nutritionally improved, reduced staling rate and acceptable injera to the consumer. As a result of the findings of this investigation, a follow-up study on the shelf life and storage materials is recommended. It is advisable to develop a starting culture and ensure that the relevant standards are met regarding these food types.
SIGNIFICANCE STATEMENT
This research work aimed to find out the maximum and modeling the flour mixing proportions of fenugreek, sorghum and teff that result in injera with higher quality attributes. The study’s findings showed that increasing the proportion of fenugreek flours in injera made from teff-sorghum-fenugreek mixing ratios improved nutritive values, improved sensory appeal, textural characteristics and reduced staling rate. This could ultimately help to avail nutritionally improved, reduced staling rate and acceptable injera to the consumer.
ACKNOWLEDGEMENTS
We thank the staff of the Kulumsa, Melkassa and Debrezeit Agricultural research centers and Addis Ababa University for providing the teff, sorghum and fenugreek products, their rounded facilitation and execution of experimental work.
REFERENCES
- Batool, R., M.S. Butt, M.T. Sultan, F. Saeed and R. Naz, 2015. Protein-energy malnutrition: A risk factor for various ailments. Crit. Rev. Food Sci. Nutr., 55: 242-253.
- Yetneberk, S., L.W. Rooney and J.R.N. Taylor, 2005. Improving the quality of sorghum injera by decortication and compositing with tef. J. Sci. Food Agric., 85: 1252-1258.
- Bultosa, G., A.N. Hall and J.R.N. Taylor, 2002. Physico-chemical characterization of grain tef [Eragrostis tef (Zucc.) Trotter] starch. Starch/Stärke, 54: 461-468.
- Girma, T., G. Bultosa and N. Bussa, 2013. Effect of grain tef [Eragrostis tef (Zucc.) Trotter] flour substitution with flaxseed on quality and functionality of injera. Int. J. Food Sci. Technol., 48: 350-356.
- Fox, G., Y. Nugusu, H. Nida, T. Tedessa, G. McLean and D. Jordan, 2020. Evaluation of variation in Ethiopian sorghum injera quality with new imaging techniques. Cereal Chem., 97: 362-372.
- Abraha, A. and F. Abay, 2017. Effect of different cereal blends on the quality of injera a staple food in the highlands of Ethiopia. Momona Ethiop. J. Sci., 9: 232-241.
- Neela, S. and S.W. Fanta, 2020. Injera (an ethnic, traditional staple food of Ethiopia): A review on traditional practice to scientific developments. J. Ethnic Foods, 7.
- Bultosa, G., 2016. Teff: Overview. In: Encyclopedia of Food Grains, Wrigley, C., H. Corke, K. Seetharaman and J. Faubion (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780123947864, pp: 209-220.
- Zegeye, A., 1997. Acceptability of injera with stewed chicken. Food Qual. Preference, 8: 293-295.
- Yetneberk, S., H.L. de Kock, L.W. Rooney and J.R.N. Taylor, 2004. Effects of sorghum cultivar on injera quality. Cereal Chem., 81: 314-321.
- Wani, S.A. and P. Kumar, 2018. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci., 17: 97-106.
- Yigzaw, Y., L. Gorton, T. Solomon and G. Akalu, 2004. Fermentation of seeds of teff (Eragrostis teff), Grass-pea (Lathyrus sativus), and their mixtures: Aspects of nutrition and food safety. J. Agric. Food Chem., 52: 1163-1169.
- Awulachew, M.T., 2021. Black cumin (Nigella sativa L.): A review on effect and scientific developments in animal and human ailments. Int. J. Med. Biotechnol. Genet., 8: 64-72.
- Visuvanathan, T., L.T.L. Than, J. Stanslas, S.Y. Chew and S. Vellasamy, 2022. Revisiting Trigonella foenum-graecum L.: Pharmacology and therapeutic potentialities. Plants, 11.
- Ranpariya, M.G. and R.S. Chudasama, 2010. Evaluation of total phenols and antibacterial activity of certain drug plants against some bacterial species. J. Appl. Nat. Sci., 2: 140-144.
- Legassa, O., T. Kore, M. Seifu and D. Mengistu, 2022. Improvement of injera quality through incorporation of fenugreek in tef flour. Sci. Dev., 3: 104-109.
- Leykun, T., S. Admasu and S. Abera, 2020. Evaluation of the mineral content, phyto-chemicals profile and microbial quality of tef injera supplemented by fenugreek flour. J. Food Sci. Technol., 57: 2480-2489.
- Woldemariam F., A. Mohammed, T.F. Teferra and H. Gebremedhin, 2019. Optimization of amaranths-teff-barley flour blending ratios for better nutritional and sensory acceptability of injera. Cogent Food Agric., 5.
- Tura, D.C., T. Belachew, D. Tamiru and K.H. Abate, 2023. Optimization of dabi teff-field pea based energy and protein dense novel complementary food with improved sensory acceptability using D-optimal mixture design. Heliyon, 9.
- Awulachew, M.T., 2020. Evaluation of proximate composition and sensory quality acceptability of Ethiopian flat bread (Injera) prepared from composite flour, blend of maize, teff and sorghum. Int. J. Food Eng. Technol., 4: 18-24.
- >Burns, R.E., 1971. Method for estimation of tannin in grain sorghum. Agron. J., 63: 511-512.
- Maxson, E.D. and L.W. Rooney, 1972. Evaluation of methods for tannin analysis in sorghum grain. Cereal Chem., 49: 719-728.
- Marolt, G. and M. Kolar, 2021. Analytical methods for determination of phytic acid and other inositol phosphates: A review. Molecules, 26.
- Licciardello, F., L. Cipri and G. Muratore, 2014. Influence of packaging on the quality maintenance of industrial bread by comparative shelf life testing. Food Packag. Shelf Life, 1: 19-24.
- Parra, V., J. Viguera, J. Sánchez, J. Peinado, F. Espárrago, J.I. Gutierrez and A.I. Andrés, 2010. Modified atmosphere packaging and vacuum packaging for long period chilled storage of dry-cured Iberian ham. Meat Sci., 84: 760-768.
- Cherie, Z., G.R. Ziegler, H.F. Gemede and A.Z. Woldegiorgis, 2018. Optimization and modeling of teff-maize-rice based formulation by simplex lattice mixture design for the preparation of brighter and acceptable injera. Cogent Food Agric., 4.
- Yegrem, L., S. Abera and M. Temesgen, 2021. Nutritional composition and sensory quality of injera prepared from tef (Eragrostis tef (Zucc.) Trotter) complemented with lupine (Lupinus spp.). Cogent Food Agric., 7.
- Sowmya, P. and P. Rajyalakshmi, 1999. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Food Hum. Nutr., 53: 359-365.
- Tasie, M.M. and B.G. Gebreyes, 2020. Characterization of nutritional, antinutritional, and mineral contents of thirty-five sorghum varieties grown in Ethiopia. Int. J. Food Sci., 2020.
- Ashenafi, M., 2006. Review article: A review on the microbiology of indigenous fermented foods and beverages of Ethiopia. Ethiopian J. Biol. Sci., 5: 189-245.
- Abraha, A., A.K. Uhlen, F. Abay, S. Sahlstrøm and A. Bjørnstad, 2013. Genetic variation in barley enables a high quality, the Ethiopian staple flat bread, comparable to Tef. Crop Sci., 53: 2040-2050.
- Khorshidian, N., M.Y. Asli, M. Arab, A.A. Mirzaie and A.M. Mortazavian, 2016. Fenugreek: Potential applications as a functional food and nutraceutical. Nutr. Food Sci. Res., 3: 5-16.
- Agza, B., R. Bekele and L. Shiferaw, 2018. Quinoa (Chenopodium quinoa, Wild.): As a potential ingredient of injera in Ethiopia. J. Cereal Sci., 82: 170-174.
- Adetunji, A.I., K.G. Duodu and J.R.N. Taylor, 2015. Inactivation of tannins in milled sorghum grain through steeping in dilute NaOH solution. Food Chem., 175: 225-232.
- Fischer, M.M., I.M. Egli, I. Aeberli, R.F. Hurrell and L. Meile, 2014. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int. J. Food Microbiol., 190: 54-60.
- Gómez, M., F. Ronda, C.A. Blanco, P.A. Caballero and A. Apesteguía, 2003. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol., 216: 51-56.
- Calle, J., Y. Benavent-Gil and C.M. Rosell, 2020. Development of gluten free breads from Colocasia esculenta flour blended with hydrocolloids and enzymes. Food Hydrocolloids, 98.
- Blandino, A., M.E. Al-Aseeri, S.S. Pandiella, D. Cantero and C. Webb, 2003. Cereal-based fermented foods and beverages. Food Res. Int., 36: 527-543.
- Ghebrehiwot, H.M., H.A. Shimelis, K.P. Kirkman, M.D. Laing and T. Mabhaudhi, 2016. Nutritional and sensory evaluation of injera prepared from tef and Eragrostis curvula (Schrad.) Nees. Flours with sorghum blends. Front. Plant Sci., 7.
- Pandey, H. and P. Awasthi, 2015. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J. Food Sci. Technol., 52: 1054-1060.
- Cox, D.R., 1971. A note on polynomial response functions for mixtures. Biometrika, 58: 155-159.
- Black, R.E., L.H. Allen, Z.A. Bhutta, L.E. Caulfield and M. de Onis et al., 2008. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371: 243-260.
How to Cite this paper?
APA-7 Style
Awulachew,
M.T., Kuffi,
K.D. (2024). Improving the Quality of Injera by Optimizing the Mixing Proportion of Fenugreek, Sorghum and Teff Flour
. Asian Science Bulletin, 2(4), 535-554. https://doi.org/10.3923/asb.2024.535.554
ACS Style
Awulachew,
M.T.; Kuffi,
K.D. Improving the Quality of Injera by Optimizing the Mixing Proportion of Fenugreek, Sorghum and Teff Flour
. Asian Sci. Bul 2024, 2, 535-554. https://doi.org/10.3923/asb.2024.535.554
AMA Style
Awulachew
MT, Kuffi
KD. Improving the Quality of Injera by Optimizing the Mixing Proportion of Fenugreek, Sorghum and Teff Flour
. Asian Science Bulletin. 2024; 2(4): 535-554. https://doi.org/10.3923/asb.2024.535.554
Chicago/Turabian Style
Awulachew, Melaku, Tafese, and Kumsa Delessa Kuffi.
2024. "Improving the Quality of Injera by Optimizing the Mixing Proportion of Fenugreek, Sorghum and Teff Flour
" Asian Science Bulletin 2, no. 4: 535-554. https://doi.org/10.3923/asb.2024.535.554

This work is licensed under a Creative Commons Attribution 4.0 International License.




