Physiological Responses of Ornamental Plants Grown on Crude Oil-Contaminated Soil
| Received 17 Jan, 2025 |
Accepted 15 Mar, 2025 |
Published 31 Mar, 2025 |
Background and Objective: Soil contamination by crude oil disrupts plant growth and physiology, yet the physiological responses of ornamental plants under such stress remain poorly understood. Physiological reactions of ornamental plants grown on crude oil-contaminated soil were investigated. Materials and Methods: Eighteen polythene bags were filled with 20 kg of soil, arranged at 0.5 m spacing between polybags and 1 m between replications, and perforated at the base to avoid water logging. Unpolluted soil served as control without any treatments. The crude oil concentrations were 0, 15, 30, 45 and 60%. The experiment was laid out in a completely randomized design with 3 replicates. Growth parameters such as plant height, number of leaves, stem girth, and leaf length were measured fortnightly and analyzed using SPSS Version 20. The data were analyzed using ANOVA in SPSS (Version 17), and significant differences were determined by Tukey’s test at a 5% probability level. Results: It showed that adding crude oil altered the physicochemical properties of the experimental soil 2 weeks after spiking. Results obtained showed that there was a significant difference (p<0.05) between heavy metal concentrations in crude oil-polluted soil and the control (unpolluted experimental soils). The changes indicate a slight increase in the concentration of TPH from 22.65 to 21470.67 mg/kg in the 2nd week of the study. The mean plant height of Periwinkle (Catharanthus roseus) was significantly (p<0.05) high at 8 WAP (7.4±1.9 cm) in control while the lowest mean concentration was recorded from 60% treatment (2.9±1.1 cm). Conclusion: Similarly, mean plant girth, number of leaves, and length were significantly high in the control compared to treated soil. The highest mean value of growth parameters in Acalypha indica, Horsetails (Equisetum arvense), and Eranthemum laxiflorum was recorded from the control while the lowest values were recorded from 60% treatment.
INTRODUCTION
Soils are a rich ecological system comprising both abiotic and biotic matter with varying levels of interaction and provision of ecosystem services1. Globally, human societies are interconnected economies that rely on services provided by the ecosystems, which constitute the foundation upon which human existence is based. The resilience of socio-ecological systems solely depends on the sustainable management of these ecosystems, hence, the need for a sustainable eco-friendly management strategy to curb the menace of environmental contamination2.
Over the years, Nigeria has experienced environmental pollution of different forms (land, water, air) and efforts have been made by both governmental and non-governmental organizations in finding a lasting solution to this menace3. Across the length and breadth of the country, the influx of companies (locals and multinationals) engaged in all manner of activities impacts negatively on our environment4. The management of hydrocarbon is an essential environmental management tool, especially in mining companies that deal with large volumes of hydrocarbons and hydrocarbon-related wastes5. Soil contamination with petroleum hydrocarbons is a result of oil excavation and shipping and is a possible threat to agriculture production. Soil contaminated with oil is a concern because the contaminated soils are not suitable for agricultural, industrial, or recreational uses and are possible sources of surface and groundwater contamination6. Agriculture happens to be the major source of food in Nigeria and all over the world, is hindered because of this contamination. Through the expanding population of the country, shortages of food could lead to poor health and poor standards of living.
Current public concern and rising costs regarding conventional clean-up procedures demonstrate the need for a less expensive bioremediation option such as phytoremediation. Bioremediation is largely considered a promising technology for the tropics because climatic conditions favor microbial growth and activity. One of the first steps in the selection of species for phytoremediation in the tropics is the screening of plant species for their capability to grow and establish in contaminated soil, followed by the evaluation of their influence on the degradation of petroleum hydrocarbons in soil7. Thus, this research aimed at evaluating the efficiency of using ornamental plants in the reclamation of crude oil-polluted agricultural soil.
MATERIALS AND METHODS
Description of study area: The study was carried out from 12 August, to 15 December, 2024, at the Screen house of the Department of Plant Science and Biotechnology, Imo State University Owerri located at Latitude 5.3866°N, and Longitude 6.9916°E.
Collection of soil and plant samples: Soil sample (sandy-loam) was collected from fallow farmland close to the Botanical Garden of Imo State University. The soil was mixed to get a homogenous mixture. Crude oil was acquired from Onne in Port Harcourt, Rivers State. Seedlings of ornamental plant species used in this study (Eranthemum pulchellum, Catharanthus roseus, Acalypha indica, Equisetum arvense) were collected from Okonkon horticultural services, Rivers State, and sent to the Taxonomy Unit of the Department of Plant Science and Biotechnology for proper identification and authentication before use.
Method of pre-soil analysis: The following physicochemical parameters were determined for the present study as follows.
Particle size distribution: It was determined by the hydrometer method8. The result from particle size distribution was reported as a percentage of sand, silt, and clay, respectively.
Soil moisture retention: Water retention was determined by the hanging water column technique9. The hanging water column procedure involved the collection of undisturbed core soil samples. The metal cores used had dimensions of 4.8 cm (internal diameter, ID) and 5.6 cm in height. After saturation for 24 hrs, the cores were used to measure soil water retention at a matric potential of -6 kPa and then oven-dried at 105°C for 24 hrs. Soil water content was measured gravimetrically.
Soil pH: Determination of the soil was in duplicates both in distilled water and in 0.1 N KCl solution, using a soil/water ratio of 1:2.5. After stirring for 30 min, the pH values were read off using a Beckman zeromatic pH meter10.
Total nitrogen: The total nitrogen was determined using the micro-Kjeldahl distillation method11. The ammonia from the digestion was distilled with 45% NaOH into 2.5% boric acid and determined by titrating with 0.05 N KCl.
Available phosphorus: Available phosphorus was determined by the Bray-2 method. This method involved weighing 2 g of soil sample into a test tube. Then, 20 ml of 0.03 NH4F in 0.1 NHCl was added to the sample of soil in the test tube. The test tube was closed and shaken for a minute. It was allowed to settle and filtered with 608 filter paper. About 1 mL of the filtrate was pipetted into a 50 mL volumetric flask. Then, 7 mL of distilled water, 1 mL of NH4 molybdate and 1 mL of ascorbic acid were added to the sample. The flask was made up to the mark with distilled water and allowed to stand for 15 min before taking the reading. The available phosphorus was read off from the standard curve obtained from optical density using a colorimeter.
Organic carbon: Organic carbon determination was done by using the old method12. The percentage organic matter was calculated by multiplying the value for organic carbon by the “Van Bemmelen factor” of 1.724, which is based on the assumption that soil organic matter (SOM) contains 58% C (Allison, 1982).
Exchangeable bases: Calcium (Ca) and Magnesium (Mg) were determined by titration method12. Sodium (Na) and Potassium (K) were extracted with 1N Ammonium Acetate Solution (NH4OAC), and determined using a flame photometer3.
Soil organic matter: It was determined by calculation5:
Where:
1.724 = Correlation factor (Van Bemmelen factor, which is constant)
Heavy metal determination: Heavy metals were determined using the double acid method of extraction and extraction acid read out with AAS. The samples were mixed gently, homogenized, and sieved through a 2 mm mesh sieve. The samples were first dried, and then placed in an electric oven at a temperature of 40°C approximately for 30 min. The resulting fine powder was kept at room temperature for digestion.
Digestion of soil samples: About 1 g of the oven-dried sample was weighed using a top loading balance and placed in 250 mL beakers separately to which 15 mL of aqueous solutions (35% HCl, and 70% high purity HNO3 in 3:1 ratio) was added. The mixture was then digested at 70% till the solution became transparent. The resulting solution was filtered through Whatman filter paper no 42 and into a 50 mL dilute 50 mL volumetric flask and diluted to mark volume using deionized water and the sample solution was analyzed for concentration of Cd, Pb, Cr, Fe, As, and Zn using an atomic absorption spectrophotometer (Perkin-Elmer AAnalyst 400).
Determination of TPH content: The TPH content of the soil samples was extracted11-14. About 10 g of the soil sample was carefully mixed with 150 mL dichloromethane, which was used as the extraction solvent and extracted for 4 hrs 30 min. This was done in the presence of 2.5 g of dried sodium sulfate and 300 μg/mL of 1-chloro-octadecane as a surrogate standard. About 0.3 g of silica was introduced into the extraction mixture after the extraction to facilitate the adsorption of polar materials like animal fats and oil from vegetable materials. The extracts were later passed through a Whatman glass fiber filter for filtration. The materials not removed by silica gel such as oil and grease were considered PHs3. The separation and determination of TPH contained in the soil samples were carried out with Gas
Chromatography equipped with a Flame Ionization Detector (GC-FID) (Agilent 6890N). A concentrated 3 μL of the sample was introduced into the GC column with a micro-syringe previously rinsed with dichloromethane (blank) and the sample. The TPH was determined at a specific chromatogram in mg/kg.
Pot experiments: Phytoremediation potentials of selected ornamental plant species were evaluated in vivo by conducting pot experiments. Soil samples were collected from unpolluted sites at a depth of 0-20 cm using an auger of approximately 7.5 cm diameter and taken to the laboratory for pre-planting analysis. The soil samples were sieved with a <2 mm sieve and used for routine soil analysis.
Experimental design/layout and treatment application: Eighteen polythene bags were filled with 20 kg of soil, arranged at 0.5 m spacing between polybags and 1 m between replications, and perforated at the base to avoid water logging. Unpolluted soil served as control without any treatments. The crude oil concentrations were 0, 15, 30, 45 and 60%. The setup was left for 2 weeks before the introduction of the ornamental plants. The experiment was laid out in a completely randomized design with 3 replicates. The effect of crude oil pollution at various concentration levels was evaluated on the various morphological parameters of the selected ornamental plant species along with their bioaccumulation potentials in the tissues (roots, stem, and leaf). The observations were made on the morphology of the plant, growth rate, and metal and TPH bioaccumulation.
Determination of growth parameters of test plants: The following plant growth traits were recorded for each of the ornamental plants as follows:
| • | Plant height (cm): This was measured with the aid of the meter rule. The perpendicular distance from the ground level to the tip of the longest branch was measured on three randomly selected plants and the average data was recorded in cm | |
| • | Stem girth (cm): This was measured with a micrometer gauge | |
| • | Leaf length (cm) and leaf numbers: Leaf length was measured with a meter rule while leaf number was estimated by counting |
At 12 weeks after planting (WAP), soil analysis was done to determine the remaining heavy metal concentration in the soil to determine the percentage of contamination reduction. In the final week of the experiment (12 weeks after planting), soil and plant (root and shoot) samples were again analyzed to determine the residual heavy metal concentration. The WHO’s (2016) permissible limit for heavy metal concentration in the soil and plant was used as a standard and as a rating for each ornamental plant’s phytoremediation potential.
Shoot dry weight (SDW) (g): The weight of the above-ground dry biomass of the three randomly selected shoots will be recorded after the above-ground biomass was oven-dried for 24 hrs at 70 and the average data was recorded.
Statistical analysis: The data were subjected to Analysis of Variance (ANOVA) using Statistical Package for Social Sciences (Version 17). Significant differences were found between each other using the Tukey’s Test at 5% level of probability.
RESULTS
Physicochemical analysis: The results obtained from the physicochemical analysis of the experimental soil before and after pollution with crude oil are presented in Table 1. The results showed that adding crude oil altered the physicochemical properties of the experimental soil 2 weeks after spiking as seen in Table 1. Crude oil pollution led to a decline in soil pH, total nitrogen (TN), and available phosphorus (AP), with pH dropping from 5.32 (control) to 4.03 at 60% pollution. Organic carbon (OC) increased, peaking at 3.76 in the highest contamination level. Essential nutrients like Ca, K, and Mg showed slight reductions, while soil texture remained sandy loam (SL) across treatments. The results indicate that crude oil contamination alters soil chemical properties, potentially affecting plant growth.
| Table 1: | Physicochemical properties of crude oil polluted soils before and after soil amendment | |||
| Treatments | pH | OC | TN | AP | Ca | K | Mg | TC | Sand | Silt | Clay |
| Before soil pollution | |||||||||||
| T1 (0%) | 5.32 | 2.68 | 0.021 | 18.16 | 0.42 | 0.12 | 0.22 | SL | 82.96 | 5 | 5.02 |
| 2 weeks after crude oil pollution | |||||||||||
| T1 (15%) | 5.17 | 2.69 | 0.02 | 18.15 | 0.41 | 0.11 | 0.22 | SL | 78.22 | 5.07 | 4.72 |
| T2 (30%) | 5.02 | 3.35 | 0.021 | 16.82 | 0.36 | 0.1 | 0.22 | SL | 82.96 | 4.09 | 5.06 |
| T3 (45%) | 4.21 | 3.53 | 0.012 | 13.13 | 0.34 | 0.11 | 0.22 | SL | 78.45 | 4.24 | 4.67 |
| T4 (60%) | 4.03 | 3.76 | 0.015 | 6.46 | 0.33 | 0.1 | 0.21 | SL | 78.27 | 4.1 | 4.68 |
| OC: Organic carbon, TN: Total nitrogen, AP: Available phosphorus, Ca: Calcium, TC: Textural class, K: Potassium, Mg: Magnesium and SL: Sandloam | |||||||||||
| Table 2: | Heavy metal contents of experimental soil before and after crude oil pollution | |||
| Heavy metals in mg/kg (DPR limit) | |||||
| Treatments | Pb (0.3) | Cd (0.2) | Ni (<10) | As (<5) | Cr ( 0.5) |
| Before soil pollution | |||||
| 0% | ND | ND | ND | ND | ND |
| After soil pollution | |||||
| 15% | 2.1 | 0.7 | 13.6 | 4.4 | 4.3 |
| 30% | 2 | 1.1 | 17.8 | 4.6 | 4.5 |
| 45% | 2.2 | 1.3 | 17.7 | 4.8 | 5.4 |
| 60% | 2.8 | 1.5 | 18 | 6.1 | 5.8 |
| Mean | 2.9 | 1.2 | 16.8 | 4.8 | 5.0 |
| C.V (%) | 0.8 | 0.4 | 1.9 | 1.1 | 0.7 |
| Pb: Lead, Cd: Cadmuim, Ni: Nickel, As: Arsenic, Cr: Chromium and C.V: Coefficient of variation | |||||
Heavy metal contents of experimental soil before and after crude oil pollution: Results of the Heavy metal concentration of the experimental soil before and after crude oil pollution with the corresponding DPR Standards is shown in Table 2. Heavy metals assayed were Lead (Pb), Cadmium (Cd), Arsenic (As), Chromium (Cr) and Zinc (Zn). Results obtained showed that there was a significant difference (p<0.05) between heavy metal concentrations in crude oil polluted soil and the control (unpolluted experimental soils). It was also revealed that there was no trace of metals detected in the experimental soil used for the study; however, 2 weeks after crude oil pollution of the experimental soil, traces of heavy metals were detected as shown in Table 2. Heavy metal concentrations in the soil increased significantly after pollution, exceeding DPR limits. The Pb, Cd, Ni, As, and Cr levels rose with higher contamination levels, with Pb peaking at 2.8 mg/kg and Ni at 18.0 mg/kg in the 60% treatment. The mean values for all metals surpassed safe thresholds, highlighting severe contamination.
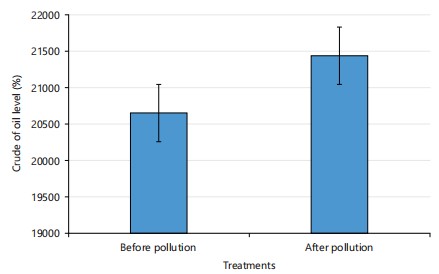
TPH concentration before and after crude oil pollution: Results also showed changes in the TPH contents of the soil before and after pollution. The changes indicate a slight increase in the concentration of TPH from 22.65 to 21470.67 mg/kg on the 2nd week of the study as shown in Fig. 1.
Growth parameters of Catharanthus roseus, Acalypha indica, Equisetum arvense and Eranthemum laxiflorum grown on crude oil contaminated soil: Table 3 shows the results on growth parameters of Catharanthus roseus, Acalypha indica, Equisetum arvense and Eranthemum laxiflorum grown on crude oil-contaminated soil is presented in Table 3. The growth parameters such as plant height (cm), plant girth (cm), number of leaves (cm), and leaf length (cm) were measured at various concentrations per plant. The results revealed that there was a significant difference (p<0.05) in the growth parameters measured with respect to treatments. There was a dose dependent decrease in growth parameters of the ornamental plant species. Although the experimental plants had optimum growth rates in crude oil polluted soils, the Mean plant height (cm), plant girth (cm), number of leaves (cm) and leaf length (cm) were higher in controls when compared with other treatment combinations.
|
| Table 3: | Mean growth parameters of Periwinkle (Catharanthus roseus), Acalypha indica, Horsetails (Equisetum arvense), and Eranthemum laxiflorum at 8 WAP | |||
| Treatments (concentration) | Plant height (cm) | Plant girth (cm) | Number of leaves (cm) | Leaf length |
| Periwinkle (Catharanthus roseus) | ||||
| T0 (control) | 7.4±1.9b | 3.4±0.4b | 4.3±0.8b | 3.7±0.7b |
| T1 (15%) | 4.2±1.2c | 2.1±0.1c | 3.4±0.5c | 2.5±0.4c |
| T2 (30%) | 4.1±1.4c | 2.2±0.4c | 3.5±0.6c | 2.6±0.5c |
| T3 (45%) | 3.9±1.1c | 2.0±0.2c | 3.0±0.3c | 2.4±0.5c |
| T4 (60%) | 2.9±1.1c | 1.0±0.2c | 2.0±0.3c | 1.7±0.6c |
| Acalypha indica | ||||
| T1 (15%) | 8.7±1.8a | 4.3±0.8a | 5.6±1.0a | 4.3±0.9a |
| T2 (30%) | 9.2±1.9a | 4.4±0.9a | 5.4±0.9a | 4.7±0.8a |
| T3 (45%) | 8.5±1.7a | 4.3±0.8a | 5.5±1.0a | 4.2±0.8a |
| T4 (60%) | 3.9±1.1c | 2.0±0.2c | 3.0±0.3c | 2.7±0.6c |
| Horsetails (Equisetum arvense) | ||||
| T1 (15%) | 15.6±2.5a | 6.2±1.2a | 8.1±2.6a | 7.6±2.4a |
| T2 (30%) | 16.0±2.6a | 6.3±1.3a | 8.4±2.8a | 8.0±2.5a |
| T3 (45%) | 16.1±2.6a | 5.9±1.1a | 8.2±2.7a | 6.9±2.7a |
| T4 (60%) | 03.5±1.1c | 2.1±0.3c | 3.0±0.3c | 2.4±0.6c |
| Eranthemum laxiflorum | ||||
| T1 (15%) | 5.2±1.5c | 3.3±0.4c | 4.7±0.9c | 3.7±0.8c |
| T2 (30%) | 5.3±1.4c | 3.0±0.4c | 4.5±0.8c | 4.0±0.7c |
| T3 (45%) | 5.1±1.3c | 3.3±0.4c | 4.7±0.9c | 4.0±0.8c |
| T4 (60%) | 3.9±1.1c | 2.0±0.2c | 3.0±0.3c | 2.7±0.2c |
| Mean along the column having different superscript of letters differ significantly at p = 0.05 level according to Duncan’s Multiple Range Test (DMRT) | ||||
The mean plant height of Periwinkle (Catharanthus roseus) was significantly (p<0.05) high at 8 WAP (7.4±1.9 cm) in control while the lowest mean concentration was recorded from 60% treatment (2.9±1.1 cm). Similarly, mean plant girth, number of leaves, and length were significantly higher in control compared to treated soil.
The highest mean value of growth parameters in Acalypha indica, Horsetails (Equisetum arvense), and Eranthemum laxiflorum was recorded from the control while the least values were recorded from 60% treatment. However, the results revealed that four species used for the study grew well in crude oil-polluted soil in a dose-dependent manner.
DISCUSSION
The results from the crude contaminated soils showed that the crude oil altered the physicochemical properties of the soils, and sparingly influenced the growth parameters at various concentrations. Previous researchers had reported effects of crude oil pollution on soil physicochemical parameters3,6.
There was no consistency in the growth performance as severe impact was felt on the number of leaves, stem girth, and leaf number of the test plants planted in soils polluted with different concentrations of crude oil. The severe impact felt on the test plants planted on the crude oil polluted soil could be attributed to excess levels of metals, which may have inhibited physiologically active enzymes as earlier speculated11. It was observed that the growth parameters of the test plants decreased with a corresponding increase in crude oil treatments on soil. This corroborated the view of Okoh5 who earlier observed that crude oil pollution reduces the growth and yield of plantain and root crops as a result of depletion in soil fertility in the Niger Delta Region of Nigeria.
This study focused on a limited duration and specific plant species, which may not fully capture long-term physiological responses to crude oil contamination. Future research should explore a broader range of plant species, extended monitoring periods, and the integration of soil amendments to enhance phytoremediation efficiency. Field-based studies are also recommended to validate the effectiveness of these plants in real-world contaminated environments.
CONCLUSION
This study demonstrated that crude oil contamination significantly altered the physicochemical properties of the soil and negatively affected plant growth. The tested plant species, including Catharanthus roseus, Acalypha indica, Equisetum arvense, and Eranthemum laxiflorum, exhibited resilience in crude oil-polluted soil, though their growth parameters, such as plant height, girth, number of leaves, and length, were significantly reduced compared to the control. The ability of these plants to survive in contaminated environments highlights their potential for phytoremediation, offering a sustainable approach to soil recovery while maintaining environmental aesthetics.
SIGNIFICANCE STATEMENT
This study highlights the impact of crude oil contamination on soil properties and plant growth, demonstrating the resilience of selected ornamental plant species in polluted environments. Despite significant reductions in growth parameters, the ability of Catharanthus roseus, Acalypha indica, Equisetum arvense, and Eranthemum laxiflorum to survive in crude oil-laden soils underscores their potential for phytoremediation. These findings suggest that integrating such plants into contaminated sites could aid in soil rehabilitation while enhancing environmental aesthetics.
REFERENCES
- Azorji, J.P.N., A.C. Udebuani, C.E. Igwe, M.O Nwachukwu, C.U. Nwachukwu, P.O. Nzenwa and M.C. Igbokwe, 2023. Phytoaccumulation potentials of three indigenous plant species grown in used engine oil-polluted soil. Fac. Nat. Appl. Sci. J. Sci. Innovations, 5: 17-29.
- Azorji, J.P.N., C.E. Igwe, P.O. Nzenwa and M.C. Igbokwe, 2024. Remediation of spent engine oil polluted soil using indigenous plant species. Asian Sci. Bull., 2: 435-444.
- Nnawuike, A.J.P., A.C. Udebuani, N.U. Chibuike, I.C. Ekene, D.K. Stanley, N.P. Odinaka and I.M. Chukwuebuka, 2024. Ecological risk evaluation of spent engine oil pollution using earthworm and microbial bioassays. Sustinere: J. Environ. Sustainability, 8: 91-102.
- Njoku, C., C.N. Mbah, O. Elom and J.O. Agwu, 2021. Effect of mechanic village activities on selected soil properties in Abakaliki Southeastern Nigeria. J. Agric. Ecol. Res. Int., 22: 10-16.
- Okoh, A.I., 2003. Biodegradation of bonny light crude oil in soil microcosm by some bacterial strains isolated from crude oil flow stations saver pits in Nigeria. Afr. J. Biotechnol., 2: 104-108.
- Olayinka, O.O., H.O. Adedeji, A.A. Akinyemi and O.J. Oresanya, 2017. Assessment of the pollution status of Eleyele Lake, Ibadan, Oyo State, Nigeria. J. Health Pollut., 7: 51-62.
- Udebuani, A.C., C.G. Okoli, I.C. Okoli, H.C. Nwigwe and P.T.E. Ozoh, 2011. Assessments of the volume and disposal methods of spent engine oil generated in Nekede Mechanic Village, Owerri, Nigeria. Rep. Opin., 3: 31-36.
- Udebuani, A.C., C.I. Okoli, H. Nwigwe and P.T.E. Ozoh, 2011. Effects of spent engine oil pollution on arable soil of Nekede Mechanic Village Owerri, Nigeria. Int. J. Nat. Appl. Sci., 7: 257-260.
- Yahaya, S.M., F. Abubakar and N. Abdu, 2021. Ecological risk assessment of heavy metal-contaminated soils of selected villages in Zamfara State, Nigeria. SN Appl. Sci., 3.
- Onwurah, I.N.E., V.N. Ogugua, N.B. Onyike, A.E. Ochonogor and O.F. Otitoju, 2007. Crude oil spills in the environment, effects and some innovative clean-up biotechnologies. Int. J. Environ. Res., 1: 307-320.
- Okafor, E.C. and K. Opuene, 2007. Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in the sediments. Int. J. Environ. Sci. Technol., 4: 233-240.
- Orji, C.N., F.W. Abdulrahman and N.R. Isu, 2018. Pollution status of heavy metals in spent oil-contaminated soil in Gwagwalada. Asian J. Appl. Chem. Res., 1.
- Oseni, O.M., O.E. Dada and A.A. Adelusi, 2015. Bioaccumulation potentials of Momordica charantia L. medicinal plant grown in lead polluted soil under organic fertilizer amendment. Notulae Sci. Biol., 7: 289-294.
- Okonokhua, B.O., B. Ikhajiagbe, G.O. Anoliefo and T.O. Emede, 2007. The effects of spent engine oil on soil properties and growth of maize (Zea mays L.). J. Appl. Sci. Environ. Manage., 11: 147-152.
How to Cite this paper?
APA-7 Style
Chukwuebuka,
I.M., Chikaodi,
O.H., Ngozi,
A.J. (2025). Physiological Responses of Ornamental Plants Grown on Crude Oil-Contaminated Soil. Asian Science Bulletin, 3(1), 111-118. https://doi.org/10.3923/asb.2025.111.118
ACS Style
Chukwuebuka,
I.M.; Chikaodi,
O.H.; Ngozi,
A.J. Physiological Responses of Ornamental Plants Grown on Crude Oil-Contaminated Soil. Asian Sci. Bul 2025, 3, 111-118. https://doi.org/10.3923/asb.2025.111.118
AMA Style
Chukwuebuka
IM, Chikaodi
OH, Ngozi
AJ. Physiological Responses of Ornamental Plants Grown on Crude Oil-Contaminated Soil. Asian Science Bulletin. 2025; 3(1): 111-118. https://doi.org/10.3923/asb.2025.111.118
Chicago/Turabian Style
Chukwuebuka, Igbokwe, Moses, Ogbuehi, Hyginus Chikaodi, and Akalazu, Jacinta Ngozi.
2025. "Physiological Responses of Ornamental Plants Grown on Crude Oil-Contaminated Soil" Asian Science Bulletin 3, no. 1: 111-118. https://doi.org/10.3923/asb.2025.111.118

This work is licensed under a Creative Commons Attribution 4.0 International License.




