Synergistic Antimicrobial Activities and Phytochemical Analysis of Leaf Extracts from Sarcocephalus latifolius, Morinda lucida and Anogeissus leiocarpus
| Received 23 Feb, 2024 |
Accepted 14 May, 2024 |
Published 31 Dec, 2024 |
Background and Objective: Morinda lucida (ML), Anogeissus leiocarpus (AL) and Sarcocephalus latifolius (SL) have long been valued in traditional medicine for their therapeutic properties. In Nigeria, a decoction of their leaves is commonly utilized to address various health issues, including lymphatic filariasis, often complicated by wounds or sores. The present study aimed to assess the phytochemical profile and synergistic antimicrobial activity of leaf extracts from Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius. Materials and Methods: The extracts of ML, AL, SL and a combination thereof were prepared via decoction and subjected to phytochemical analysis and high-performance liquid chromatography. The extracts were further evaluated for their antioxidant potential against DPPH and antimicrobial effects against a range of pathogens. Results: Phytochemical analysis unveiled the presence of bioactive compounds like saponins, terpenes, sterols, phenolics and tannins. The high-performance liquid chromatography elucidated the specific chemical profiles of each extract, with ML featuring prominent peaks of betulinic acid, chlorogenic acid, caffeic acid, rutin and ferulic acid. The AL showcased major peaks of gallic acid, chlorogenic acid and caffeic acid, while SL exhibited significant peaks of betulinic acid, gallic acid, caffeic acid and rutin. The combination extract displayed peaks corresponding to these compounds. Conclusion: Remarkably, AL demonstrated the highest antimicrobial and antioxidant activities, followed by the combination extract. These findings offer promising insights into the therapeutic potential of combined herbal therapy for lymphatic filariasis, warranting further investigation.
INTRODUCTION
Medicinal plants have been used as sources of drugs by mankind for several years. In fact, ancient man was totally dependent on plants for his needs of treatment, prevention and other forms of medicaments, thus utilizing plants as drugs for millennia1. For the past 3000 years, a large number of plants are used in health care practices, such as Traditional Medicine in China, India and Africa, most of which contain therapeutic values which have been ascertained as such by the Western standards2. Furthermore, several other plants have been employed for centuries by several cultures which are less likely to be proven by Western standards. Medicinal plants however remain a major source of novel drug discovery and development and have contributed greatly to human health. Nature’s pharmacy has bestowed humans with an endless supply of plants enriched with various secondary metabolites for man to harness. Plants have provided an endless array of chemicals for man since the beginning of man’s existence. Several oxidation reactions take place in the biological system resulting in oxidative stress, one of such is the univalent reduction of oxygen which results in the conversion of a certain percentage of inhaled oxygen into reactive species or free radicals called reactive oxygen species (ROS)3. Antioxidants are also called free radical scavengers and are compounds whose presence even at low concentrations possess the ability to prevent oxidation of cell constituents like lipid, carbohydrate, DNA and protein; they act via a number of processes such as by preventing the formation of free radicals through preventing phagocyte activation; through binding of transition metal ions and prevention of hydroxyl formation and via repair of damage as in tocopherol which repairs peroxyl radicals thereby halting lipid peroxidation and preventing decomposition of lipid hydroperoxide4.
A decoction made from the leaves of Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius is commonly used in Nigeria as a traditional remedy for treating lymphatic filariasis, even in cases complicated by wounds or sores5,6. Morinda lucida, scientifically known as Benth and belonging to the Rubiaceae family, is referred to as Brimstone tree in English, Erhan Ikpanro in Benin, Huka or Eze ogu in Igbo and Oruwo in Yoruba7. This evergreen tree has a crooked or gnarled appearance, with leaves that are opposite, simple and entire8. The bark ranges from smooth to roughly scaly, while the wood is yellow in color9,10. Morinda lucida has a rich history of safe consumption and has been reported to possess trypanocidal and antimalarial activities11.
Anogeissus leiocarpus, belonging to the Combretaceae family, is known by various vernacular names such as African birch, Bambara, Anogeissus and chewstick12. This graceful tree is native to the savannahs of tropical Africa and is characterized by its slow growth and grey to mottled pale and dark brown bark13. It has alternate to nearly opposite leaves covered in dense silky hair when young. Anogeissus leiocarpus is utilized in various traditional medicinal practices across Africa, including Nigeria, where it is used for treating coughs and maintaining dental hygiene13.
Sarcocephalus latifolius, a member of the Rubiaceae family, is known by names such as African Peach, Pin cushion tree and Guinea peach8. This savannah tree or shrub can grow up to 12 meters high and is characterized by its twisted bole and spreadingly open crown12. The fruits of Sarcocephalus latifolius are fused together into a fleshy mass and the seeds are embedded in pinkish flesh with a strawberry scent. This plant is widely distributed throughout Africa and Asia and is reputedly used for treating malaria and other ailments14.
The present study aimed to assess the phytochemical profile and synergistic antimicrobial activity of leaf extracts from Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius. These plants have a long history of traditional use in managing various diseases and their combined use reflects the rich heritage of herbal medicine in Nigeria and other regions of Africa.
MATERIALS AND METHODS
Collection and identification of plant sample: Fresh leaves of Morinda lucida Benth, Anogeissus leiocarpus (D.C) Guill and Perr and Sarcocephalus latifolius J E Smith (E A Bruce) were harvested from the NIPRD garden and authenticated by Mr. Akeem Lateef, an expert taxonomist, at the herbarium of the National Institute for Pharmaceutical Research and Development in Abuja, Nigeria. This study was carried out from February, 2019 to July, 2020. Voucher specimens with the numbers NIPRD/H/7038, NIPRD/H/7039 and NIPRD/H/7040 were deposited for reference. The plant materials were then air-dried at room temperature for two weeks and subsequently ground into a fine powder using an electric grinder.
Preparation of plant extracts: The air-dried leaves were chopped into smaller pieces and weighed, with each plant material weighing 300 g for Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius. Additionally, a combination therapy consisting of 100 g each of Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius was prepared. The extraction process involved boiling the chopped leaves with distilled water for 10-15 min, using 750 mL of water, followed by allowing the mixture to stand overnight for 24 hrs. Afterward, the extracts were filtered using a funnel blocked with cotton wool. The resulting filtrates were then dried over a water bath to obtain dark brown extracts, with percentage yields of 3.3, 4.9 and 4.8% w/w, respectively.
Phytochemical analysis: Phytochemical analysis was conducted on the extracts for secondary metabolites and using standard methods of Wakawa et al.15.
High performance liquid chromatography analysis: The analysis of bioactive constituents in the extract was conducted using high-performance liquid chromatography (HPLC) with a UV diode array detector (UV-DAD). The HPLC setup included an Ultra-Fast LC-20AB equipped with a SIL-20AC auto-sampler, DGU-20A3 degasser, SPD-M20A UV-diode array detector, column oven CTO-20AC, system controller CBM-20Alite and Windows LC solution software, all from Shimadzu Corporation in Kyoto, Japan. The column used was a 5μm VP-ODS C18 with dimensions of 4.6×150 mm.
Chromatographic conditions were as follows: The mobile phase consisted of 0.2% v/v formic acid and acetonitrile in a ratio of 20:80; the mode was isocratic; the flow rate was set at 0.6 mL/min; 10 μL of a 100 mg/mL solution of the extract in water was injected; detection was performed at UV 254 nm. The HPLC operating conditions were programmed to maintain solvent B at 20% and the column oven temperature was set to 40°C. The total run time for the analysis was 30 min. For the identification of phytoconstituents, flavonoids and phenolic acid standards such as apigenin, rutin, quercetin, caffeic acid and ferulic acid were used, with their retention times compared under similar experimental conditions16.
Antimicrobial and antioxidant evaluations
Test organisms: Pure clinical isolates of Bacillus subtilis, Klebsiella pneumoniae, collected and biochemically confirmed from Diagnostic Laboratory of NIPRD clinic and American Type cultures of Escherichia coli (ATCC25952), Staphylococcus aureus (ATCC25923), Pseudomonas aeruginosa (ATCC 27853), Salmonella paratyphi (ATCC 9150), Mycobacterium bovis (27290) and Mycobacterium smegmatis (607) were used in this study.
Preparation of inoculum: A loopful of the respective test organisms (S. aureus, E. coli, P. aeruginosa, B. subtilis, K. pneumoniae and S. paratyphi) was aseptically transferred from the agar slants to 5 mL of nutrient broth and then placed in an incubator at 37°C. After 24 hrs of incubation, the cultures were diluted with normal saline to achieve turbidity equivalent to a 0.5 McFarland standard (106 CFU/mL) as described by Adamu et al.17.
The 50 microliters (50 μL) of each freshly thawed stock test organism (M. bovis and M. smegmatis) was inoculated into 50 mL of sterile Middlebrook 7H9/ADC media and then incubated at 37ºC with shaking for 5-7 days. The actively growing cultures of M. bovis and M. smegmatis were adjusted to an optical density between 0.2-0.3 at a wavelength of 650 nm using a Jenway 6405 UV-Visible spectrophotometer.
Antimicrobial activity of samples: Concentrations of each sample (100 mg/mL) were prepared and tested against the test organisms using the Agar well diffusion method. One hundred microliters (100 μL) of each standardized microorganism suspension was evenly spread on sterile molten Mueller Hinton agar in Petri dishes, which were then allowed to solidify. Aseptically, holes were bored using a sterile cork borer (6 mm) and the bottoms of the holes were sealed with a drop of Mueller Hinton agar. Then, 100 μL of each sample concentration were dispensed into the labeled wells. The plates were left to dry in a biosafety cabinet for approximately 2 hrs to allow the samples to diffuse. Subsequently, they were incubated at 37°C for 24-48 hrs. After incubation, antimicrobial activity was evaluated by measuring the zone of inhibition around each well and the average of the readings from duplicate plates was calculated18.
Antimicrobial activity: The antimicrobial test of the samples was carried out using the broth micro-dilution method in 96-well microtiter plates to determine the minimum inhibitory concentration (MIC). Each sample (500 mg) was dissolved in 5 mL of sterile water to obtain a stock concentration of 100 mg/mL, which was then serially diluted across the microtiter plate in two-fold dilutions. Duplicate assays were performed for each extract concentration. Mueller Hinton broth (50 μL) was dispensed into sterile wells of the microtiter plate from rows 1 to 12. Subsequently, 50 μL of the 100 mg/mL concentration of the samples was transferred into well 1 of the plate in duplicate. The contents of well 1 were then mixed thoroughly and 50 μL was transferred to well 2 and this process was repeated sequentially up to well 11, where 50 μL was discarded. Each of the wells (1-12) was inoculated with 50 μL of diluted organisms and incubated for 24 hrs at 37°C. After the dilution procedure and incubation, the final testing concentrations ranged from 25 to 0.0977 mg/mL. Following incubation, 25 μL of tetrazolium salt dye was added to all the wells and the plate was reincubated for 1 hrs. The wells were then observed for the absence or presence of microbial growth by examining the color change in the wells. The MIC was defined as the lowest concentration of the drug/extract that prevented the tetrazolium dye from changing color to pink. A colorless well indicated no microbial growth, while a pink color indicated the occurrence of growth19,20.
Antioxidant activity
Free radical scavenging assays: The 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) assay is a widely used method to assess the radical scavenging capacity of antioxidant compounds. This assay relies on the reduction of DPPH, a stable free radical, in an alcohol solution when exposed to hydrogen-donating species, such as antioxidant compounds. This reduction results in the formation of the non-radical form of DPPH, known as DPPH-H, leading to a color change from purple to yellow, which can be quantified using a spectrophotometer. The disappearance of the purple color is measured at 517 nm. For the assay, a reaction mixture was prepared consisting of 1.0 mL of 0.3 mM DPPH in methanol, 1.0 mL of various concentrations of the extract (250, 125, 62.5, 31.25 and 15.625 μg/mL) and 1.0 mL of methanol. This mixture was then incubated for 10 minutes in the dark, following which the absorbance was measured at 517 nm using a spectrophotometer. Ascorbic acid was used as a positive control in this assay21.
Statistical analysis: Statistical analysis was carried out with the statistical package BMDP, using the BMDP 2R program (stepwise multiple regression). Results were expressed as mean of triplicate analysis.
RESULTS AND DISCUSSION
Table 1, presented the extractive values of Morinda lucida, Anogeissus leiocarpus, Sarcocephalus latifolius and their combination therapy. Extract weight in grams and yield percentage (w/w) were provided for each plant and combination therapy.
| Table 1: | Extractive values of the plants singly and combination therapy | |||
| Plant | Extract weight (g) | Yield % (w/w) |
| Morinda lucida | 9.8273 | 3.3 |
| Anogeissus leiocarpus | 14.7117 | 4.9 |
| Sarcocephalus latifolius |
14.3595 | 4.8 |
| Combination therapy | 13.589 | 4.5 |
| Table 2: | Phytochemical analysis of the plants singly and combination therapy | |||
| Extract | ||||
| Secondary metabolites | Morinda lucida | Anogeissus leiocarpus | Sarcocephalus latifolius | Combination therapy |
| Carbohydrates | Positive | Positive | Positive | Positive |
| Tannins | Negative | Positive | Positive | Positive |
| Saponins | Positive | Positive | Positive | Positive |
| Terpenes | Positive | Positive | Positive | Positive |
| Sterols | Positive | Positive | Positive | Positive |
| Flavonoids | Positive | Positive | Positive | Positive |
| Alkaloids | Negative | Negative | Negative | Negative |
| Anthraquinones | Negative | Negative | Negative | Negative |
| Table 3: | Antimicrobial activity of samples at 100 mg/mL concentration against microbial isolates zone of inhibition (mm) | |||
| Extract | Streptococcus pyogenes |
Staphylococcus aureus |
Salmonella paratyphi |
Escherichia coli |
Pseudomonas aeruginosa |
Klebsiella pneumoniae |
Bacillus subtilis |
Candida albicans |
| Mixed | 17 | 17 | 15 | 10.5 | 13 | 9.5 | 14.5 | 20 |
| AL | 19 | 19.5 | 17.5 | 12.5 | 17.5 | 16 | 17 | 20.5 |
| SL | 9.5 | - | - | - | - | - | - | 9 |
| ML | - | - | - | - | - | - | - | - |
| Microorganisms: Streptococcus pyogenes, Staphylococcus aureus, Salmonella paratyphi, Escherichia coli, Pseudomonas aeruginosa, Klebsiella puemoniae, Bacillis subtillis and Candida albicans. ML: Morinda lucida, SL: Sarcocephalus latifolius, AL: Anogeissus leiocarpus and MIX: Combination therapy | ||||||||
Anogeissus leiocarpus gave the highest extractive value of 4.9% (w/w), followed by Anogeissus leiocarpus 4.8% (w/w) and the combination therapy 4.5% (w/w) as shown in Table 1.
Table 2, outlined the results of phytochemical analysis for Morinda lucida, Anogeissus leiocarpus, Sarcocephalus latifolius and their combination therapy. It indicated the presence or absence of secondary metabolites such as carbohydrates, tannins, saponins, terpenes, sterols, flavonoids, alkaloids and anthraquinones in each extract.
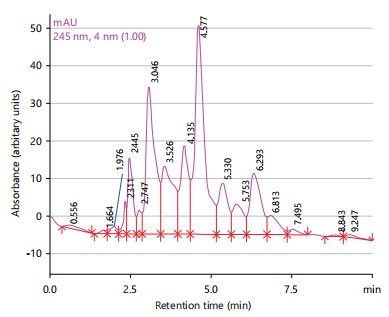
Figure 1 displayed the High-Performance Liquid Chromatography (HPLC) spectrum of Morinda lucida extract showed thirteen peaks with major peaks being betulinic acid (3.526 min), chlorogenic acid (4.135 min), caffeic acid (4.577 min), rutin (6.293 min) and ferulic acid (7.495 min).
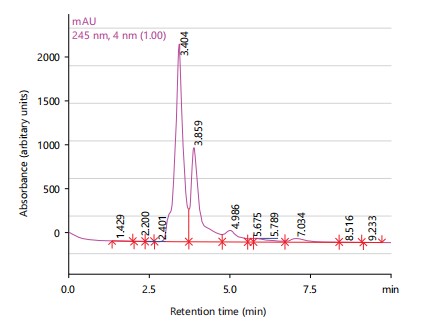
The HPLC spectrum of Anogeissus leiocarpus extract showed four peaks with major peaks being gallic acid (3.404 min), chlorogenic acid (3.859 min) and caffeic acid (4.986 min) as shown in Fig. 2.
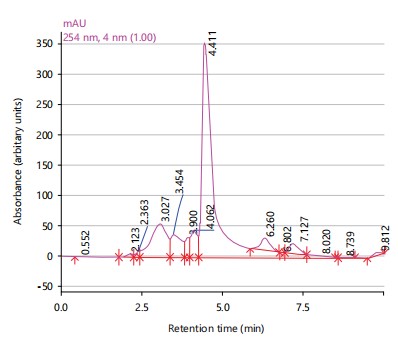
The HPLC spectrum of Sarcocephalus latifolius extract showed six peaks with major peaks being betulinic acid (2.123 min), gallic acid (3.454 min), caffeic acid (4.411 min) and rutin (6.260 min) as shown in Fig. 3.
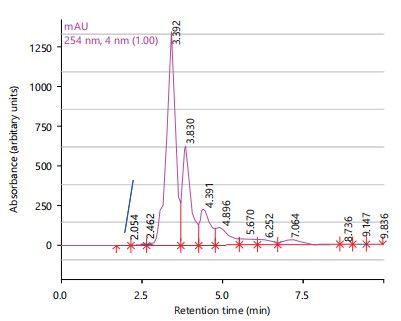
The HPLC spectrum of the combination therapy showed eight peaks with major peaks being betulinic acid (2.462 min), gallic acid (3.392 min), chlorogenic acid (3.830 min), caffeic acid (4.391 min) and rutin (6.252 min) as shown in Fig. 4.
Table 3, presented the antimicrobial activity of the extracts against various microbial isolates at a concentration of 100 mg/mL. The zone of inhibition (in millimeters) for Streptococcus pyogenes,
|
|
Staphylococcus aureus, Salmonella paratyphi, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Bacillus subtilis and Candida albicans was provided for each sample.
Table 4, displayed the Minimum Inhibitory Concentration (MIC) of the extracts against selected microbial isolates. The MIC values in micrograms per milliliter (μg/mL) were listed for Streptococcus pyogenes, Staphylococcus aureus, Salmonella paratyphi, Escherichia coli, Pseudomonas aeruginosa, Klebsiella puemoniae, Bacillus subtilis and Candida albicans.
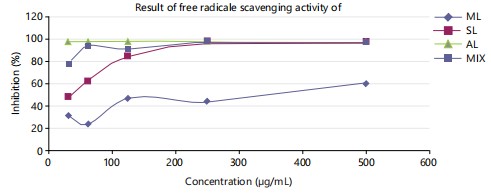
Figure 5 illustrated the DPPH free radical scavenging activities of Morinda lucida, Sarcocephalus latifolius, Anogeissus leiocarpus and the combination therapy. Key abbreviations ML, SL, AL and MIX represent Morinda lucida, Sarcocephalus latifolius, Anogeissus leiocarpus and combination therapy, respectively.
|
|
| Table 4: | Minimum inhibitory concentration of samples against selected microbial isolates MIC (μg/mL) | |||
| Sample | Streptococcus pyogenes |
Staphylococcus aureus |
Salmonella paratyphi |
Escherichia coli |
Pseudomonas aeruginosa |
Klebsiella pneumoniae |
Bacillus subtilis |
Candida albicans |
| Combinations therapy | 1560 | 1560 | 1560 | 3125 | 6250 | 3125 | 1560 | 195.3 |
| Anogeissus leiocarpus | 781.3 | 195.3 | 781.3 | 1560 | 3125 | 1560 | 781.3 | 97.7 |
| Sarcocephalus latifolius | 6250 | NA | NA | 6250 | 6250 | 12500 | 12500 | 6250 |
| Morinda lucida | NA | NA | NA | NA | NA | NA | NA | NA |
| NA: No activity | ||||||||
Anogeissus leiocarpus gave the highest extractive value of 4.9% (w/w), followed by Anogeissus leiocarpus 4.8% (w/w) and the combination therapy 4.5% (w/w) as shown in Table 1.
Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius are well-known medicinal plants with extensive traditional use in Nigeria for treating various ailments22. A combination of their leaves, prepared as a decoction, is commonly used as an herbal remedy for lymphatic filariasis and associated complications like wounds or sores23,24.
|
The extracts from the leaves of these plants were obtained through decoction and subjected to phytochemical analysis using standard methods and high-performance liquid chromatography (HPLC). Additionally, the extracts were evaluated individually and in combination for their antioxidant activity using the DPPH assay and their antimicrobial effects against a range of pathogens including Streptococcus pyogenes, Staphylococcus aureus, Salmonella paratyphi, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Bacillus subtilis and Candida albicans24.
Phytochemical analysis revealed the presence of various compounds such as saponins, terpenes, sterols, phenolics and tannins in the plant extracts24. The HPLC analysis further identified specific compounds in each plant extract, with major peaks corresponding to known antioxidants and bioactive compounds. Anogeissus leiocarpus extract exhibited the highest antimicrobial and antioxidant activities, followed by the combination of the three plants25. These findings support the traditional use of the combination therapy for treating lymphatic filariasis, particularly regarding its antimicrobial and wound-healing properties25.
Furthermore, studies on Morinda lucida and Sarcocephalus latifolius have highlighted their antibacterial, antifungal, anti-inflammatory and antimalarial properties26. The phytochemical constituents of Sarcocephalus latifolius include carbohydrates, reducing sugars, tannins, saponins, flavonoids, alkaloids, steroids, glycosides, terpenoids and phenols26. Sarcocephalus latifolius has been traditionally used to treat a variety of conditions such as fevers, pains, dental issues, hypertension, dysentery, diarrhea and neurological disorders27. Additionally, essential oils extracted from Morinda lucida fruits exhibited antibacterial activity against various pathogens28.
In summary, the combined use of Morinda lucida, Anogeissus leiocarpus and Sarcocephalus latifolius shows promise in traditional medicine for managing lymphatic filariasis and associated complications, supported by their diverse pharmacological activities and phytochemical composition28,29. Further research is warranted to explore their full therapeutic potential and develop effective treatments based on these traditional remedies.
CONCLUSION
The extracts derived from the three plants and their combination exhibited a presence of carbohydrates, saponins, terpenes, flavonoids and sterols. Notably, Anogeissus leiocarpus demonstrated the highest levels of antimicrobial and antioxidant activity among the tested extracts. This research offers initial preclinical findings suggesting that the combination therapy may hold promise as a treatment for lymphatic filariasis (elephantiasis), pending further clinical investigations.
SIGNIFICANCE STATEMENT
The study aimed to evaluate the phytochemical profile and synergistic antimicrobial activity of Morinda lucida (ML), Anogeissus leiocarpus (AL) and Sarcocephalus latifolius (SL) leaf extracts, commonly used in Nigerian traditional medicine for addressing health issues like lymphatic filariasis. Phytochemical analysis revealed bioactive compounds such as saponins, terpenes, sterols, phenolics and tannins. High-performance liquid chromatography identified specific chemical profiles, with ML containing betulinic acid, chlorogenic acid and others, AL featuring gallic acid, chlorogenic acid and caffeic acid and SL exhibiting betulinic acid, gallic acid and caffeic acid. AL demonstrated the highest antimicrobial and antioxidant activities, followed by the combination extract, suggesting potential therapeutic benefits in treating lymphatic filariasis.
ACKNOWLEDGEMENT
The authors wish to thank the Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Abuja, Nigeria, for providing technical support for this study.
REFERENCES
- Imoisi, C., J.U. Iyasele and S.E. Okhale, 2021. Proximate and acute toxicity profile of Vitex doniana (black plum) fruit. J. Chem. Soc. Nigeria, 46: 276-282.
- Okhale, S.E. and C. Imoisi, 2022. Characterization of the volatile bioactive compounds in ethylacetate leaf extract of Annona muricata Linn. Life Sci. J., 19: 57-62.
- Okhale, S.E., H.O. Egharevba, C. Imoisi, J.A. Ibrahim and I.A. Jegede, 2022. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of the essential oil from Nigerian Artemisia annua L. at different growth stages. Nat. Sci., 20: 49-54.
- Okhale, S.E., N. Amuzie, C. Imoisi and J.A. Ibrahim, 2022. Phytochemical and HPLC-UV-DAD chromatographic characterization of stem bark extracts of Pentaclethra macrophylla Benth used for management of diabetes mellitus in Nigeria. New York Sci. J., 15: 41-49.
- Imoisi, C., J.U. Iyasele and S.A. Okhale, 2021. Phytochemical and antioxidant capability of Vitex doniana (black plum) fruit. J. Chem. Soc. Nigeria, 46: 191-196.
- Egbeneje, V.O., S.E. Okhale, C. Imoisi, I.O. Ogbogo and O. Ojo, 2023. Evaluation of the inhibitive properties of silver nanoparticles in Senna occidentalis root extract as corrosion inhibitor of mild steel. Tanzania J. Sci., 49: 655-663.
- Imoisi, C. and U.C. Michael, 2020. Comparative physicochemical and proximate analyses of different extracts of Persea americana. J. Chem. Soc. Niger., 45: 1139-1146.
- Imoisi, C., J.U. Iyasele, E.E. Imhontu, D.O. Ikpahwore and A.O. Okpebho, 2020. Pasting properties of composite of cassava and wheat flours. J. Chem. Soc. Niger., 45: 1157-1163.
- Imoisi, C., J.U. Iyasele, U.C. Michael and E.E. Imhontu, 2020. The effects of watermelon rind flour on the functional and proximate properties of wheat bread. J. Chem. Soc. Niger., 45: 978-986.
- Ajenu, C.O., C. Imoisi, E.E. Imhontu and U.R. Orji, 2021. Comparative evaluation of the proximate and micro-nutritional benefits of pawpaw, carrot, turmeric and coconut. J. Food Technol. Nutr. Sci., 3: 1-5.
- Okhale, S.E., I. Chinyere, M.I. Aboh and U.A. Osunkwo, 2021. Effects of semisynthetic modifications on the antimicrobial activities of ethyl acetate extract of Mitracarpus villosus (Sw.) DC aerial part. Nat. Sci., 19: 36-41.
- Okhale, S.E., I. Chinyere, S.A. Fidelis and M.I. Aboh, 2021. Antiproliferative, growth inhibitory and antibacterial activities of thymol isolated from the leaf of Ocimum gratissimum L. Life Sci. J., 18: 67-76.
- Chinyere, I., I.U. Julius and O.E. Samuel, 2021. Determination of the flavoring components in Vitex doniana fruit following hydrodistillation extraction. Am. J. Food Nutr., 9: 69-75.
- Imoisi, C., J.U. Iyasele, D.O. Ikpahwore and A.O. Okpebho, 2023. The effects of watermelon rind flour on the proximate properties of wheat cake. Int. J. Nutr. Res. Health, 2.
- Wakawa, A.I., A.B. Sambo and S. Yusuf, 2018. Phytochemistry and proximate composition of root, stem bark, leaf and fruit of desert date, Balanites aegyptiaca. J. Phytopharmacol., 7: 464-470.
- Lamorde, M., J.R.S. Tabuti, C. Obua, C. Kukunda-Byobona and H. Lanyero et al., 2010. Medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in Uganda. J. Ethnopharmacol., 130: 43-53.
- Adamu, M.O., S.F. Ameh, U.A.O. Ettah and S.E. Okhale, 2018. Chromatographic and antiproliferative assessment of the aerial root of Ficus thonningii Blume (Moraceae). MicroMedicine, 6: 1-9.
- Josiah, J.G., J.Y. Adama, Z. Jiya, O.M. Abah and C. Imoisi, 2023. In vitro anthelmintic activities of stem and root barks extracts of Parkia biglobosa on infective larvae and adult of Haemonchus contortus. Afr. J. Biotechnol., 22: 26-38.
- Okhale, S.E., V.O. Egbeneje and C. Imoisi, 2021. GC-MS evaluation of palm oil as benign extraction medium for bioactive constituents of Ocimum gratissimum L and Bryophyllum pinnatum (Lam.). J. Am. Sci., 17: 46-53.
- Jayaraman, S., M.S. Manoharan and S. Illanchezian, 2008. In-vitro antimicrobial and antitumor activities of Stevia rebaudiana (Asteraceae) leaf extracts. Trop. J. Pharm. Res., 7: 1143-1149.
- Adamu, A., K.B. Esievo, G. Ugbabe, S.E. Okhale and H.O. Egharevba, 2018. High performance liquid chromatography-diode array detection (HPLC-DAD) profiling, antioxidant and anti-proliferative activities of ethanol leaf extract of Berlinia grandiflora (Vahl) Hutch. & Dalziel. J. Pharmacogn. Phytother., 10: 187-194.
- Ajenu, C.O., M.E. Ukhun, C. Imoisi, E.E. Imhontu, L.E. Irede and U.R. Orji, 2021. Characterization and stability studies of egusi melon seed oil (Citrullus colocynthis L.). J. Chem. Soc. Nigeria, 46: 238-244.
- Josiah, J.G., J.Y. Adama, E.H. Edim, I. Chinyere and J. Zipporah, 2024. Acute toxicity profile of crude methanolic stem bark extract of Parkia biglobosa in West African Dwarf (Wad) goats. J. Biosci. Biotechnol. Discovery, 9: 10-22.
- Abbah, J., S. Amos, B. Chindo, I. Ngazal and H.O. Vongtau et al., 2010. Pharmacological evidence favouring the use of Nauclea latifolia in malaria ethnopharmacy: Effects against nociception, inflammation and pyrexia in rats and mice. J. Ethnopharmacol., 127: 85-90.
- Ozoh, C.A., C. Imoisi and J.U. Iyasele, 2023. Effect of pH and duration of fermentation on the quality characteristics of garri. Pak. J. Nutr., 22: 45-51.
- Adeneye, A.A. and E.O. Agbaje, 2008. Pharmacological evaluation of oral hypoglycemic and antidiabetic effects of fresh leaves ethanol extract of Morinda lucida Benth. in normal and alloxan-induced diabetic rats. Afr. J. Biomed. Res., 11: 65-71.
- Okhale, S.E., P.O. Oladosu, M.I. Aboh, C. Imoisi and J.J. Gana, 2022. In-vitro evaluation of Eucalyptus citriodora leaf essential oil and extracts on selected pathogens implicated in respiratory tract infections. Int. J. Pharmacogn., 9: 195-201.
- Imoisi, C., J.U. Iyasele and A.O. Okpebho, 2023. The effects of citrus vesicle flour on the functional and proximate properties of cassava bread. Pak. J. Nutr., 22: 19-26.
- Chinyere, I. and I.U. Julius, 2020. Spectroscopic determination of sugar components of Vitex doniana fruit syrup following derivatization. Nat. Sci., 18: 67-76.
How to Cite this paper?
APA-7 Style
Okhale,
S.E., Imoisi,
C., Aliyu,
A., Josiah,
J.G., Egbeneje,
V. (2024). Synergistic Antimicrobial Activities and Phytochemical Analysis of Leaf Extracts from Sarcocephalus latifolius, Morinda lucida and Anogeissus leiocarpus. Asian Science Bulletin, 2(4), 425-434. https://doi.org/10.3923/asb.2024.425.434
ACS Style
Okhale,
S.E.; Imoisi,
C.; Aliyu,
A.; Josiah,
J.G.; Egbeneje,
V. Synergistic Antimicrobial Activities and Phytochemical Analysis of Leaf Extracts from Sarcocephalus latifolius, Morinda lucida and Anogeissus leiocarpus. Asian Sci. Bul 2024, 2, 425-434. https://doi.org/10.3923/asb.2024.425.434
AMA Style
Okhale
SE, Imoisi
C, Aliyu
A, Josiah
JG, Egbeneje
V. Synergistic Antimicrobial Activities and Phytochemical Analysis of Leaf Extracts from Sarcocephalus latifolius, Morinda lucida and Anogeissus leiocarpus. Asian Science Bulletin. 2024; 2(4): 425-434. https://doi.org/10.3923/asb.2024.425.434
Chicago/Turabian Style
Okhale, Samuel, Ehiabhi, Chinyere Imoisi, Adamu Aliyu, James Gana Josiah, and Victor Egbeneje.
2024. "Synergistic Antimicrobial Activities and Phytochemical Analysis of Leaf Extracts from Sarcocephalus latifolius, Morinda lucida and Anogeissus leiocarpus" Asian Science Bulletin 2, no. 4: 425-434. https://doi.org/10.3923/asb.2024.425.434

This work is licensed under a Creative Commons Attribution 4.0 International License.




